Tyrosine 51 residue of the syndecan-2 extracellular domain is involved in the interaction with and activation of pro-matrix metalloproteinase-7
- PMID: 31337828
- PMCID: PMC6650482
- DOI: 10.1038/s41598-019-47140-5
Tyrosine 51 residue of the syndecan-2 extracellular domain is involved in the interaction with and activation of pro-matrix metalloproteinase-7
Abstract
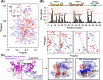
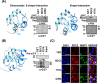
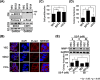
Although syndecan-2 is known to interact with the matrix metalloproteinase-7 (MMP-7), the details of their interaction were unknown. Our experiments with a series of syndecan-2 extracellular domain deletion mutants show that the interaction is mediated through an interaction of the extracellular domain of syndecan-2 (residues 41 to 60) with the α2 helix-loop-α3 helix in the pro-domain of MMP-7. NMR and molecular docking model show that Glu7 of the α1 helix, Glu32 of the α2 helix, and Gly48 and Ser52 of the α2 helix-loop-α3 helix of the MMP-7 pro-domain form the syndecan-2-binding pocket, which is occupied by the side chain of tyrosine residue 51 (Tyr51) of syndecan-2. Consistent with this notion, the expression of a syndecan-2 mutant in which Tyr51 was changed to Ala diminished the interaction between the syndecan-2 extracellular domain and the pro-domain of MMP-7. Furthermore, HT-29 colon adenocarcinoma cells expressing the interaction-defective mutant exhibited reductions in the cell-surface localization of MMP-7, the processing of pro-MMP-7 into active MMP-7, the MMP-7-mediated extracellular domain shedding of both syndecan-2 and E-cadherin, and syndecan-2-mediated anchorage-independent growth. Collectively, these data strongly suggest that Tyr51 of the syndecan-2 extracellular domain mediates its interaction with and activating processing of pro-MMP-7 and regulates MMP-7-dependent syndecan-2 functions. VSports手机版.
Conflict of interest statement
The authors declare no competing interests.
Figures




"V体育安卓版" References
Publication types
- VSports - Actions
V体育官网入口 - MeSH terms
- V体育ios版 - Actions
- "VSports app下载" Actions
- "V体育官网入口" Actions
- "V体育ios版" Actions
- VSports在线直播 - Actions
- "V体育官网" Actions
- VSports最新版本 - Actions
- "V体育安卓版" Actions
- Actions (V体育官网)
Substances
- "VSports注册入口" Actions
- "V体育平台登录" Actions
- V体育官网 - Actions
- V体育平台登录 - Actions
LinkOut - more resources
"V体育2025版" Full Text Sources

