VSports - Deoxycholic Acid-Mediated Sphingosine-1-Phosphate Receptor 2 Signaling Exacerbates DSS-Induced Colitis through Promoting Cathepsin B Release
- PMID: 29854830
- PMCID: VSports app下载 - PMC5966668
- DOI: 10.1155/2018/2481418
V体育安卓版 - Deoxycholic Acid-Mediated Sphingosine-1-Phosphate Receptor 2 Signaling Exacerbates DSS-Induced Colitis through Promoting Cathepsin B Release
V体育安卓版 - Abstract
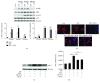
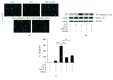
We recently have proved that excessive fecal DCA caused by high-fat diet may serve as an endogenous danger-associated molecular pattern to activate NLRP3 inflammasome and thus contributes to the development of inflammatory bowel disease (IBD). Moreover, the effect of DCA on inflammasome activation is mainly mediated through bile acid receptor sphingosine-1-phosphate receptor 2 (S1PR2); however, the intermediate process remains unclear. Here, we sought to explore the detailed molecular mechanism involved and examine the effect of S1PR2 blockage in a colitis mouse model. In this study, we found that DCA could dose dependently upregulate S1PR2 expression. Meanwhile, DCA-induced NLRP3 inflammasome activation is at least partially achieved through stimulating extracellular regulated protein kinases (ERK) signaling pathway downstream of S1PR2 followed by promoting of lysosomal cathepsin B release VSports手机版. DCA enema significantly aggravated DSS-induced colitis in mice and S1PR2 inhibitor as well as inflammasome inhibition by cathepsin B antagonist substantially reducing the mature IL-1β production and alleviated colonic inflammation superimposed by DCA. Therefore, our findings suggest that S1PR2/ERK1/2/cathepsin B signaling plays a critical role in triggering inflammasome activation by DCA and S1PR2 may represent a new potential therapeutic target for the management of intestinal inflammation in individuals on a high-fat diet. .
Figures



References
-
- Alberts D. S., Einspahr J. G., Earnest D. L., et al. Fecal bile acid concentrations in a subpopulation of the wheat bran fiber colon polyp trial. 2003;12:197–200. - PubMed
MeSH terms
- "VSports最新版本" Actions
- "V体育官网" Actions
- "VSports app下载" Actions
- Actions (V体育安卓版)
- "V体育官网入口" Actions
- Actions (VSports最新版本)
- "V体育平台登录" Actions
Substances
- "V体育平台登录" Actions
- Actions (VSports最新版本)
- "V体育ios版" Actions
- VSports app下载 - Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

