Matrix metalloproteinase (MMP)-7 in Barrett's esophagus and esophageal adenocarcinoma: expression, metabolism, and functional significance (V体育平台登录)
- PMID: 29845775
- PMCID: PMC5974721
- DOI: 10.14814/phy2.13683 (V体育ios版)
Matrix metalloproteinase (MMP)-7 in Barrett's esophagus and esophageal adenocarcinoma: expression, metabolism, and functional significance (VSports app下载)
Abstract (V体育平台登录)
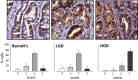
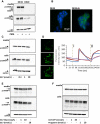
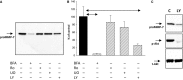
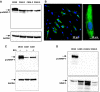
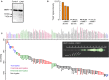
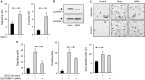
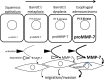
Matrix metalloproteinase (MMP)-7, unlike many MMPs, is typically expressed in epithelial cells. It has been linked to epithelial responses to infection, injury, and tissue remodeling including the progression of a number of cancers. We have now examined how MMP-7 expression changes in the progression to esophageal adenocarcinoma (EAC), and have studied mechanisms regulating its expression and its functional significance. Immunohistochemistry revealed that MMP-7 was weakly expressed in normal squamous epithelium adjacent to EAC but was abundant in epithelial cells in both preneoplastic lesions of Barrett's esophagus and EAC particularly at the invasive front. In the stroma, putative myofibroblasts expressing MMP-7 were abundant at the invasive front but were scarce or absent in adjacent tissue. Western blot and ELISA revealed high constitutive secretion of proMMP-7 in an EAC cell line (OE33) that was inhibited by the phosphatidylinositol (PI) 3-kinase inhibitor LY294002 but not by inhibitors of protein kinase C, or MAP kinase activation. There was detectable proMMP-7 in cultured esophageal myofibroblasts but it was undetectable in media. Possible metabolism of MMP-7 by myofibroblasts studied by proteomic analysis indicated degradation via extensive endopeptidase, followed by amino- and carboxpeptidase, cleavages. Myofibroblasts exhibited increased migration and invasion in response to conditioned media from OE33 cells that was reduced by MMP-7 knockdown and immunoneutralization VSports手机版. Thus, MMP-7 expression increases at the invasive front in EAC which may be partly attributable to activation of PI 3-kinase. Secreted MMP-7 may modify the tumor microenvironment by stimulating stromal cell migration and invasion. .
Keywords: Barrett's esophagus; MMP-7; PI3-kinase; myofibroblast; proteomic. V体育安卓版.
© 2018 The Authors V体育ios版. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society. .
"V体育2025版" Figures

References
-
- Adachi, Y. , Itoh F., Yamamoto H., Matsuno K., Arimura Y., Kusano M., et al. 1998. Matrix metalloproteinase matrilysin (MMP‐7) participates in the progression of human gastric and esophageal cancers. Int. J. Oncol. 13:1031–1035. - PubMed
-
- Adachi, Y. , Li R., Yamamoto H., Min Y., Piao W., Wang Y., et al. 2009. Insulin‐like growth factor‐I receptor blockade reduces the invasiveness of gastrointestinal cancers via blocking production of matrilysin. Carcinogenesis 30:1305–1313. - PubMed
-
- Beales, I. L. , and Ogunwobi O. O.. 2009. Glycine‐extended gastrin inhibits apoptosis in Barrett's oesophageal and oesophageal adenocarcinoma cells through JAK2/STAT3 activation. J. Mol. Endocrinol. 42:305–318. - PubMed
-
- Beales, I. L. , Ogunwobi O., Cameron E., El‐Amin K., Mutungi G., and Wilkinson M.. 2007. Activation of Akt is increased in the dysplasia‐carcinoma sequence in Barrett's oesophagus and contributes to increased proliferation and inhibition of apoptosis: a histopathological and functional study. BMC Cancer 7:97. - PMC - PubMed
Publication types
- "VSports app下载" Actions
MeSH terms
- Actions (V体育2025版)
- Actions (V体育安卓版)
- "V体育平台登录" Actions
- VSports最新版本 - Actions
- VSports注册入口 - Actions
- Actions (V体育2025版)
- "V体育安卓版" Actions
- V体育官网 - Actions
- V体育平台登录 - Actions
- VSports最新版本 - Actions
- V体育平台登录 - Actions
Substances
- "VSports app下载" Actions
- "VSports手机版" Actions
Grants and funding
LinkOut - more resources
"V体育官网入口" Full Text Sources
Other Literature Sources
Medical
"V体育官网入口" Research Materials
VSports最新版本 - Miscellaneous

