Animal models to study bile acid metabolism
- PMID: 29782919
- PMCID: "VSports" PMC6242766
- DOI: 10.1016/j.bbadis.2018.05.011
Animal models to study bile acid metabolism
Abstract (V体育2025版)
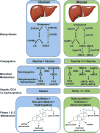
The use of animal models, particularly genetically modified mice, continues to play a critical role in studying the relationship between bile acid metabolism and human liver disease. Over the past 20 years, these studies have been instrumental in elucidating the major pathways responsible for bile acid biosynthesis and enterohepatic cycling, and the molecular mechanisms regulating those pathways. This work also revealed bile acid differences between species, particularly in the composition, physicochemical properties, and signaling potential of the bile acid pool. These species differences may limit the ability to translate findings regarding bile acid-related disease processes from mice to humans VSports手机版. In this review, we focus primarily on mouse models and also briefly discuss dietary or surgical models commonly used to study the basic mechanisms underlying bile acid metabolism. Important phenotypic species differences in bile acid metabolism between mice and humans are highlighted. .
Keywords: Enterohepatic circulation; Enzyme; Intestine; Liver; Mouse model; Transporter. V体育安卓版.
Copyright © 2018 Elsevier B. V V体育ios版. All rights reserved. .
Figures


References
-
- Chemistry, Physiology, and Metabolism. Vol. 1. New York: Plenum Press; 1971. The Bile Acids; p. 372.
-
- Beuers U, et al. New paradigms in the treatment of hepatic cholestasis: from UDCA to FXR, PXR and beyond. J Hepatol. 2015;62(1 Suppl):S25–37. - PubMed
-
- Trauner M, et al. New therapeutic concepts in bile acid transport and signaling for management of cholestasis. Hepatology. 2017;65(4):1393–1404. - "V体育官网" PubMed
Publication types
- "V体育ios版" Actions
- Actions (VSports app下载)
MeSH terms
- V体育2025版 - Actions
- V体育官网 - Actions
- Actions (VSports)
- V体育安卓版 - Actions
- "V体育ios版" Actions
Substances
- "V体育安卓版" Actions
- Actions (V体育ios版)
- Actions (V体育官网)
Grants and funding
LinkOut - more resources
"V体育官网入口" Full Text Sources
Other Literature Sources
Miscellaneous

