Curcumol potentiates celecoxib-induced growth inhibition and apoptosis in human non-small cell lung cancer
- PMID: 29383179
- PMCID: PMC5777791
- DOI: 10.18632/oncotarget.23308
"VSports app下载" Curcumol potentiates celecoxib-induced growth inhibition and apoptosis in human non-small cell lung cancer
Abstract
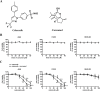
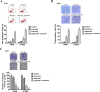
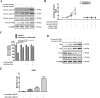
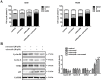
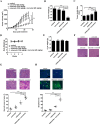
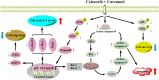
Combinatorial therapies that target multiple signaling pathways may provide improved therapeutic responses over monotherapies. Celecoxib and curcumol are two highly hydrophobic drugs which show bioavailability problems due to their poor aqueous solubility. In the present study, we evaluated the effects of celecoxib and curcumol alone and in combination on cell proliferation, invasion, migration, cell cycle and apoptosis induction in non-small cell lung cancer (NSCLC) cells using in vitro and in vivo experiments. Our data showed that the sensitivity of a combined therapy using low concentration of celecoxib and curcumol was higher than that of celecoxib or curcumol alone. Suppression of NF-κB transcriptional activity, activation of caspase-9/caspase-3, cell cycle G1 arrest, and inhibition of survival MAPK and PI3K/AKT signaling pathway contributed to the synergistic effects of this combination therapy for induction of apoptosis. Additionally, either celecoxib alone or in combination with curcumol inhibited NSCLC cell migration and invasion by suppressing FAK and matrix metalloproteinase-9 activities. Furthermore, the combined treatment reduced tumor volume and weight in xenograft mouse model, and significantly decreased tumor metastasis nodules in lung tissues by tail vein injection. Our results confirm and provide mechanistic insights into the prominent anti-proliferative activities of celecoxib and/or curcumol on NSCLC cells, which provide a rationale for further detailed preclinical and potentially clinical studies of this combination for the therapy of lung cancer. VSports手机版.
Keywords: apoptosis; celecoxib; curcumol; synergism; tumor metastasis V体育安卓版. .
"VSports" Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures



References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. - PubMed
-
- Cianchi F, Cortesini C, Bechi P, Fantappie O, Messerini L, Vannacci A, Sardi I, Baroni G, Boddi V, Mazzanti R, Masini E. Up-regulation of cyclooxygenase 2 gene expression correlates with tumor angiogenesis in human colorectal cancer. Gastroenterology. 2001;121:1339–1347. - "V体育安卓版" PubMed
-
- Dempke W, Rie C, Grothey A, Schmoll HJ. Cyclooxygenase-2: a novel target for cancer chemotherapy? J Cancer Res Clin Oncol. 2001;127:411–417. - PubMed
-
- Hida T, Yatabe Y, Achiwa H, Muramatsu H, Kozaki K, Nakamura S, Ogawa M, Mitsudomi T, Sugiura T, Takahashi T. Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res. 1998;58:3761–3764. - PubMed
LinkOut - more resources
Full Text Sources
"V体育2025版" Other Literature Sources
Research Materials
Miscellaneous

