V体育官网 - Long non-coding RNA HOTAIR acts as a competing endogenous RNA to promote malignant melanoma progression by sponging miR-152-3p
- PMID: 29156728
- PMCID: "V体育官网" PMC5689618
- DOI: 10.18632/oncotarget.19910
Long non-coding RNA HOTAIR acts as a competing endogenous RNA to promote malignant melanoma progression by sponging miR-152-3p
"V体育官网入口" Abstract
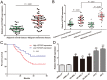
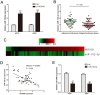
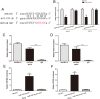
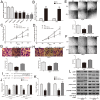
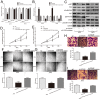
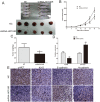
HOX transcript antisense RNA (HOTAIR) is associated with the growth and metastasis of many human tumors, but its biological roles in malignant melanoma remain unclear. In this study, we show that HOTAIR is overexpressed in melanoma tissues and cells, especially in metastatic melanoma. High HOTAIR levels correlate with poor prognosis in melanoma patients. We also determined that HOTAIR functions as a competing endogenous RNA (ceRNA) for miR-152-3p. miR-152-3p was decreased and acted as a tumor suppressor in melanoma, and c-MET was the functional target of miR-152-3p. Furthermore, HOTAIR promotes the growth and metastasis of melanoma cells by competitively binding miR-152-3p, which functionally liberates c-MET mRNA and results in the activation of the downstream PI3k/Akt/mTOR signaling pathway. We determined that HOTAIR acts as a ceRNA to promote malignant melanoma progression by sponging miR-152-3p VSports手机版. This finding elucidates a new mechanism for HOTAIR in melanoma development and provides a potential therapeutic target for melanoma patients. .
Keywords: HOTAIR; c-MET; ceRNA; malignant melanoma; miR-152-3p V体育安卓版. .
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures

References
-
- Haass NK, Schumacher U. Melanoma never says die. Exp Dermatol. 2014;23:471–472. - PubMed
-
- Millet A, Martin AR, Ronco C, Rocchi S, Benhida R. Metastatic melanoma: insights into the evolution of the treatments and future challenges. Med Res Rev. 2017;37:98–148. - PubMed
-
- Little EG, Eide MJ. Update on the current state of melanoma incidence. Dermatol Clin. 2012;30:355–361. - PubMed
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. - PubMed
-
- Russo AE, Ferrau F, Antonelli G, Priolo D, McCubrey JA, Libra M. Malignant melanoma in elderly patients: biological, surgical and medical issues. Exp Rev Anticancer Ther. 2015;15:101–108. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

