Cdk2 strengthens the intra-S checkpoint and counteracts cell cycle exit induced by DNA damage
- PMID: 29044141
- PMCID: PMC5647392
- DOI: 10.1038/s41598-017-12868-5
Cdk2 strengthens the intra-S checkpoint and counteracts cell cycle exit induced by DNA damage
V体育安卓版 - Abstract
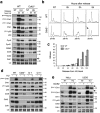
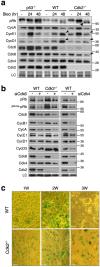
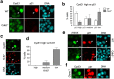
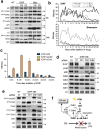
Although cyclin-dependent kinase 2 (Cdk2) controls the G1/S transition and promotes DNA replication, it is dispensable for cell cycle progression due to redundancy with Cdk1. Yet Cdk2 also has non-redundant functions that can be revealed in certain genetic backgrounds and it was reported to promote the G2/M DNA damage response checkpoint in TP53 (p53)-deficient cancer cells. However, in p53-proficient cells subjected to DNA damage, Cdk2 is inactivated by the CDK inhibitor p21. We therefore investigated whether Cdk2 differentially affects checkpoint responses in p53-proficient and deficient cell lines VSports手机版. We show that, independently of p53 status, Cdk2 stimulates the ATR/Chk1 pathway and is required for an efficient DNA replication checkpoint response. In contrast, Cdk2 is not required for a sustained DNA damage response and G2 arrest. Rather, eliminating Cdk2 delays S/G2 progression after DNA damage and accelerates appearance of early markers of cell cycle exit. Notably, Cdk2 knockdown leads to down-regulation of Cdk6, which we show is a non-redundant pRb kinase whose elimination compromises cell cycle progression. Our data reinforce the notion that Cdk2 is a key p21 target in the DNA damage response whose inactivation promotes exit from the cell cycle in G2. .
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References (V体育安卓版)
-
- Santamaria D, et al. Cdk1 is sufficient to drive the mammalian cell cycle. Nature. 2007;448:811–815. doi: 10.1038/nature06046. - "VSports在线直播" DOI - PubMed
-
- Hochegger H, et al. An essential role for Cdk1 in S phase control is revealed via chemical genetics in vertebrate cells. J Cell Biol. 2007;178:257–268. doi: 10.1083/jcb.200702034. - V体育ios版 - DOI - PMC - PubMed
-
- Ortega S, et al. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat Genet. 2003;35:25–31. doi: 10.1038/ng1232. - "V体育平台登录" DOI - PubMed
Publication types
- VSports手机版 - Actions
MeSH terms
- "VSports" Actions
- Actions (VSports在线直播)
- "V体育安卓版" Actions
- "VSports" Actions
- "V体育官网" Actions
- "V体育2025版" Actions
Substances
- Actions (VSports手机版)
- VSports - Actions
- Actions (V体育ios版)
- Actions (V体育官网)
- V体育安卓版 - Actions
- "V体育官网" Actions
- V体育安卓版 - Actions
LinkOut - more resources
Full Text Sources
VSports手机版 - Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

