Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models
- PMID: 28893978
- PMCID: PMC5635915
- DOI: 10.1073/pnas.1711235114
Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models
"V体育官网" Erratum in
-
VSports在线直播 - Correction for Cekanaviciute et al., Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models.Proc Natl Acad Sci U S A. 2017 Oct 17;114(42):E8943. doi: 10.1073/pnas.1716911114. Epub 2017 Oct 9. Proc Natl Acad Sci U S A. 2017. PMID: 29073033 Free PMC article. No abstract available.
Abstract
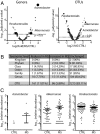
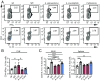
The gut microbiota regulates T cell functions throughout the body VSports手机版. We hypothesized that intestinal bacteria impact the pathogenesis of multiple sclerosis (MS), an autoimmune disorder of the CNS and thus analyzed the microbiomes of 71 MS patients not undergoing treatment and 71 healthy controls. Although no major shifts in microbial community structure were found, we identified specific bacterial taxa that were significantly associated with MS. Akkermansia muciniphila and Acinetobacter calcoaceticus, both increased in MS patients, induced proinflammatory responses in human peripheral blood mononuclear cells and in monocolonized mice. In contrast, Parabacteroides distasonis, which was reduced in MS patients, stimulated antiinflammatory IL-10-expressing human CD4+CD25+ T cells and IL-10+FoxP3+ Tregs in mice. Finally, microbiota transplants from MS patients into germ-free mice resulted in more severe symptoms of experimental autoimmune encephalomyelitis and reduced proportions of IL-10+ Tregs compared with mice "humanized" with microbiota from healthy controls. This study identifies specific human gut bacteria that regulate adaptive autoimmune responses, suggesting therapeutic targeting of the microbiota as a treatment for MS. .
Keywords: autoimmunity; microbiome; multiple sclerosis. V体育安卓版.
V体育ios版 - Conflict of interest statement
The authors declare no conflict of interest.
"VSports注册入口" Figures





Comment in
-
Multiple sclerosis: A possible link between multiple sclerosis and gut microbiota. (V体育官网入口)Nat Rev Neurol. 2017 Dec;13(12):705. doi: 10.1038/nrneurol.2017.142. Epub 2017 Sep 29. Nat Rev Neurol. 2017. PMID: 28960186 No abstract available.
-
A gut feeling about multiple sclerosis. (VSports手机版)Proc Natl Acad Sci U S A. 2017 Oct 3;114(40):10528-10529. doi: 10.1073/pnas.1714260114. Epub 2017 Sep 25. Proc Natl Acad Sci U S A. 2017. PMID: 28973867 Free PMC article. No abstract available.
References
-
- Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768–1773. - V体育官网入口 - PMC - PubMed
V体育安卓版 - Publication types
- Actions (V体育2025版)
"V体育官网" MeSH terms
- "V体育安卓版" Actions
- V体育安卓版 - Actions
- V体育安卓版 - Actions
- "VSports在线直播" Actions
- "V体育官网" Actions
- Actions (V体育2025版)
- V体育ios版 - Actions
- "V体育官网入口" Actions
- "V体育平台登录" Actions
- VSports - Actions
"V体育安卓版" Grants and funding
LinkOut - more resources
V体育ios版 - Full Text Sources
Other Literature Sources
VSports手机版 - Medical
Molecular Biology Databases
Research Materials

