Macrophage heme oxygenase-1-SIRT1-p53 axis regulates sterile inflammation in liver ischemia-reperfusion injury (V体育平台登录)
- PMID: 28842295
- PMCID: PMC5884687
- DOI: 10.1016/j.jhep.2017.08.010
Macrophage heme oxygenase-1-SIRT1-p53 axis regulates sterile inflammation in liver ischemia-reperfusion injury
V体育平台登录 - Abstract
Background & aims: Hepatic ischemia-reperfusion injury (IRI), characterized by exogenous antigen-independent local inflammation and hepatocellular death, represents a risk factor for acute and chronic rejection in liver transplantation VSports手机版. We aimed to investigate the molecular communication involved in the mechanism of liver IRI. .
Methods: We analyzed human liver transplants, primary murine macrophage cell cultures and IR-stressed livers in myeloid-specific heme oxygenase-1 (HO-1) gene mutant mice, for anti-inflammatory and cytoprotective functions of macrophage-specific HO-1/SIRT1 (sirtuin 1)/p53 (tumor suppressor protein) signaling. V体育安卓版.
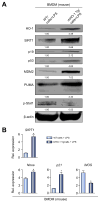
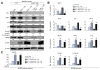
Results: Decreased HO-1 expression in human post-reperfusion liver transplant biopsies correlated with a deterioration in hepatocellular function (serum ALT; p<0. 05) and inferior patient survival (p<0. 05). In the low HO-1 liver transplant biopsy group, SIRT1/Arf (alternative reading frame)/p53/MDM2 (murine double minute 2) expression levels decreased (p<0. 05) while cleaved caspase 3 and frequency of TUNEL+cells simultaneously increased (p<0. 05). Immunofluorescence showed macrophages were the principal source of HO-1 in human and mouse IR-stressed livers. In vitro macrophage cultures revealed that HO-1 induction positively regulated SIRT1 signaling, whereas SIRT1-induced Arf inhibited ubiquitinating activity of MDM2 against p53, which in turn attenuated macrophage activation V体育ios版. In a murine model of hepatic warm IRI, myeloid-specific HO-1 deletion lacked SIRT1/p53, exacerbated liver inflammation and IR-hepatocellular death, whereas adjunctive SIRT1 activation restored p53 signaling and rescued livers from IR-damage. .
Conclusion: This bench-to-bedside study identifies a new class of macrophages activated via the HO-1-SIRT1-p53 signaling axis in the mechanism of hepatic sterile inflammation. This mechanism could be a target for novel therapeutic strategies in liver transplant recipients. VSports最新版本.
Lay summary: Post-transplant low macrophage HO-1 expression in human liver transplants correlates with reduced hepatocellular function and survival. HO-1 regulates macrophage activation via the SIRT1-p53 signaling network and regulates hepatocellular death in liver ischemia-reperfusion injury V体育平台登录. Thus targeting this pathway in liver transplant recipients could be of therapeutic benefit. .
Keywords: Heme oxygenase-1; Innate immunity; Ischemia-reperfusion injury; Liver transplantation; Myeloid-specific mutant mice; P53; Sirtuin 1 VSports注册入口. .
Copyright © 2017 European Association for the Study of the Liver. Published by Elsevier B. V. All rights reserved. V体育官网入口.
Conflict of interest statement (VSports app下载)
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Please refer to the accompanying ICMJE disclosure forms for further details.
Figures (V体育2025版)





Comment in
-
"V体育2025版" Protective effects of heme oxygenase 1 during ischemia-reperfusion injury: Hepatocytes or non-parenchymal cells?J Hepatol. 2018 Sep;69(3):752-753. doi: 10.1016/j.jhep.2018.04.028. Epub 2018 May 17. J Hepatol. 2018. PMID: 29779886 No abstract available.
-
Reply to: "Protective effects of heme oxygenase 1 during ischemia-reperfusion injury: Hepatocytes or non parenchymal cells?".J Hepatol. 2018 Sep;69(3):753-755. doi: 10.1016/j.jhep.2018.05.029. Epub 2018 Jun 2. J Hepatol. 2018. PMID: 29870762 No abstract available.
References
-
- Zhai Y, Shen XD, O’Connell R, Gao F, Lassman C, Busuttil RW, et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173:7115–7119. - PubMed
-
- Sosa RA, Zarrinpar A, Rossetti M, Lassman CR, Naini BV, Datta N, et al. Early cytokine signatures of ischemia/reperfusion injury in human orthotopic liver transplantation. JCI Insight. 2016;1:e89679. - PMC (V体育官网入口) - PubMed
-
- Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. - "V体育ios版" PubMed
Publication types (VSports)
- Actions (V体育平台登录)
MeSH terms
- "V体育平台登录" Actions
- "V体育官网" Actions
- Actions (VSports注册入口)
- VSports在线直播 - Actions
- V体育官网入口 - Actions
- Actions (V体育官网入口)
Substances
- Actions (V体育安卓版)
- Actions (VSports在线直播)
- Actions (V体育平台登录)
- "VSports app下载" Actions
V体育ios版 - Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases (V体育2025版)
Research Materials
"V体育ios版" Miscellaneous

