Farnesoid X Receptor Regulation of the NLRP3 Inflammasome Underlies Cholestasis-Associated Sepsis (V体育平台登录)
- PMID: 28380377
- PMCID: PMC6624427
- DOI: 10.1016/j.cmet.2017.03.007
Farnesoid X Receptor Regulation of the NLRP3 Inflammasome Underlies Cholestasis-Associated Sepsis
Abstract
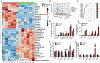
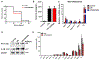
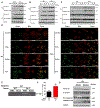
Cholestasis is a common complication of sepsis, and the increased plasma levels of bile acids are predictive of sepsis-associated mortality. However, the exact mechanism by which cholestasis aggravates sepsis development remains elusive. Here, we show that bile acids are danger-associated molecular patterns (DAMPs) that can activate both signal 1 and 2 of the NLRP3 inflammasome in inflammatory macrophages. Mechanistically, bile acids induce a prolonged calcium influx and activate the NLRP3 inflammasome synergistically with ATP. Experimental cholestasis sensitizes, while cholestyramine, a bile acid sequestrant, protects mice from LPS-induced sepsis. FXR negatively regulates the NLRP3 inflammasome via physical interaction with NLRP3 and caspase 1. Fxr-null mice are more sensitive, while FXR-overexpressing mice are more resistant, to endoxemia shock. These findings suggest that bile acids and FXR play pivotal roles in sepsis via controlling the NLRP3 inflammasome, and that targeting FXR may represent a therapeutic strategy for cholestasis-associated sepsis. VSports手机版.
Keywords: NLRP3 inflammasome; bile acids; cholestasis; damage-associated molecular patterns; farnesoid X receptor; protein-protein interaction; sepsis. V体育安卓版.
Copyright © 2017 Elsevier Inc V体育ios版. All rights reserved. .
Figures




Comment in
-
A Novel Protective Role for FXR against Inflammasome Activation and Endotoxemia.Cell Metab. 2017 Apr 4;25(4):763-764. doi: 10.1016/j.cmet.2017.03.014. Cell Metab. 2017. PMID: 28380370
-
V体育ios版 - Sepsis: Bile acids promote inflammation in cholestasis-associated sepsis.Nat Rev Gastroenterol Hepatol. 2017 Jun;14(6):324-325. doi: 10.1038/nrgastro.2017.55. Epub 2017 Apr 21. Nat Rev Gastroenterol Hepatol. 2017. PMID: 28428635 No abstract available.
References
VSports app下载 - MeSH terms
- V体育官网入口 - Actions
- "VSports手机版" Actions
- Actions (VSports app下载)
- "VSports app下载" Actions
Substances
- Actions (VSports app下载)
- "VSports注册入口" Actions
- Actions (V体育官网入口)
- V体育平台登录 - Actions
- "V体育ios版" Actions
Grants and funding
LinkOut - more resources (VSports)
Full Text Sources
Other Literature Sources
Medical
"VSports最新版本" Molecular Biology Databases

