VSports手机版 - Outcomes of acute leukemia patients transplanted with naive T cell-depleted stem cell grafts
- PMID: 26053664
- PMCID: PMC4563691
- DOI: V体育官网入口 - 10.1172/JCI81229
VSports最新版本 - Outcomes of acute leukemia patients transplanted with naive T cell-depleted stem cell grafts
Abstract
Background: Graft-versus-host disease (GVHD) is a major cause of morbidity and mortality following allogeneic hematopoietic stem cell transplantation (HCT) VSports手机版. In mice, naive T cells (TN) cause more severe GVHD than memory T cells (TM). We hypothesized that selective depletion of TN from human allogeneic peripheral blood stem cell (PBSC) grafts would reduce GVHD and provide sufficient numbers of hematopoietic stem cells and TM to permit hematopoietic engraftment and the transfer of pathogen-specific T cells from donor to recipient, respectively. .
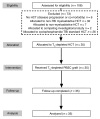
Methods: In a single-arm clinical trial, we transplanted 35 patients with high-risk leukemia with TN-depleted PBSC grafts following conditioning with total body irradiation, thiotepa, and fludarabine. GVHD prophylactic management was with tacrolimus immunosuppression alone. Subjects received CD34-selected PBSCs and a defined dose of TM purged of CD45RA+ TN V体育安卓版. Primary and secondary objectives included engraftment, acute and chronic GVHD, and immune reconstitution. .
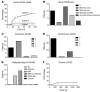
Results: All recipients of TN-depleted PBSCs engrafted. The incidence of acute GVHD was not reduced; however, GVHD in these patients was universally corticosteroid responsive. Chronic GVHD was remarkably infrequent (9%; median follow-up 932 days) compared with historical rates of approximately 50% with T cell-replete grafts V体育ios版. TM in the graft resulted in rapid T cell recovery and transfer of protective virus-specific immunity. Excessive rates of infection or relapse did not occur and overall survival was 78% at 2 years. .
Conclusion: Depletion of TN from stem cell allografts reduces the incidence of chronic GVHD, while preserving the transfer of functional T cell memory. VSports最新版本.
Trial registration: ClinicalTrials. gov (NCT 00914940) V体育平台登录. .
Trial registration: ClinicalTrials. gov NCT00914940 VSports注册入口. .
Figures





VSports - References
Publication types
MeSH terms
- Actions (VSports注册入口)
- "VSports" Actions
- "VSports最新版本" Actions
- Actions (VSports注册入口)
- "VSports最新版本" Actions
- Actions (VSports在线直播)
- "VSports在线直播" Actions
- VSports在线直播 - Actions
- VSports手机版 - Actions
- "VSports app下载" Actions
- VSports注册入口 - Actions
Substances
- VSports app下载 - Actions
Associated data
- Actions (VSports)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

