Oncogenic Role of the Ec Peptide of the IGF-1Ec Isoform in Prostate Cancer
- PMID: 25569803
- PMCID: PMC4461578 (VSports手机版)
- DOI: 10.2119/molmed.2014.00222
Oncogenic Role of the Ec Peptide of the IGF-1Ec Isoform in Prostate Cancer
Abstract
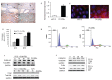
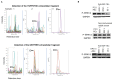
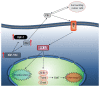
IGF-1 is one of the key molecules in cancer biology; however, little is known about the role of the preferential expression of the premature IGF-1 isoforms in prostate cancer. We have examined the role of the cleaved COO- terminal peptide (PEc) of the third IGF-1 isoform, IGF-1Ec, in prostate cancer. Our evidence suggests that endogenously produced PEc induces cellular proliferation in the human prostate cancer cells (PC-3) in vitro and in vivo, by activating the ERK1/2 pathway in an autocrine/paracrine manner. PEc overexpressing cells and tumors presented evidence of epithelial to mesenchymal transition, whereas the orthotopic injection of PEc-overexpressing, normal prostate epithelium cells (HPrEC) in SCID mice was associated with increased metastatic rate. In humans, the IGF-1Ec expression was detected in prostate cancer biopsies, where its expression correlates with tumor stage. Our data describes the action of PEc in prostate cancer biology and defines its potential role in tumor growth, progression and metastasis. VSports手机版.
VSports手机版 - Figures




References
-
- Gilmour RS. The implications of insulin-like growth factor mRNA heterogeneity. J Endocrinol. 1994;140:1–3. - PubMed
-
- Delany AM, Pash JM, Canalis E. Cellular and clinical perspectives on skeletal insulin-like growth factor I. J Cell Biochem. 1994;55:328–33. - PubMed (VSports app下载)
-
- Dai Z, Wu F, Yeung EW, Li Y. IGF-IEc expression, regulation and biological function in different tissues. Growth Horm IGF Res. 2010;20:275–81. - "VSports" PubMed
-
- Armakolas A, et al. Preferential expression of IGF-1Ec (MGF) transcript in cancerous tissues of human prostate: evidence for a novel and autonomous growth factor activity of MGF E peptide in human prostate cancer cells. Prostate. 2010;70:1233–42. - PubMed
Publication types
- V体育平台登录 - Actions
VSports在线直播 - MeSH terms
- V体育2025版 - Actions
- "V体育官网入口" Actions
- V体育2025版 - Actions
- V体育官网 - Actions
- Actions (V体育官网)
- Actions (VSports app下载)
- V体育官网入口 - Actions
- V体育官网入口 - Actions
- Actions (V体育官网入口)
- Actions (V体育官网)
- VSports在线直播 - Actions
- V体育安卓版 - Actions
- V体育官网入口 - Actions
- Actions (VSports在线直播)
Substances
- Actions (V体育安卓版)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
"VSports在线直播" Miscellaneous

