The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity
- PMID: 25212991
- PMCID: PMC4692801
- DOI: 10.1158/2326-6066.CIR-14-0127
The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity
Abstract
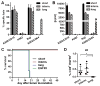
The use of synthetic chimeric antigen receptors (CAR) to redirect T cells to recognize tumor provides a powerful new approach to cancer immunotherapy; however, the attributes of CARs that ensure optimal in vivo tumor recognition remain to be defined. Here, we analyze the influence of length and composition of IgG-derived extracellular spacer domains on the function of CARs. Our studies demonstrate that CD19-CARs with a long spacer from IgG4 hinge-CH2-CH3 are functional in vitro but lack antitumor activity in vivo due to interaction between the Fc domain within the spacer and the Fc receptor-bearing myeloid cells, leading to activation-induced T-cell death. We demonstrate that in vivo persistence and antitumor effects of CAR-T cells with a long spacer can be restored by modifying distinct regions in the CH2 domain that are essential for Fc receptor binding VSports手机版. Our studies demonstrate that modifications that abrogate binding to Fc receptors are crucial for CARs in which a long spacer is obligatory for tumor recognition as shown here for a ROR1-specific CAR. These results demonstrate that the length and composition of the extracellular spacer domain that lacks intrinsic signaling function can be decisive in the design of CARs for optimal in vivo activity. .
©2014 American Association for Cancer Research V体育安卓版. .
Conflict of interest statement (VSports app下载)
Conflicts of interest: M. H. , M. C. J. , and S. R. R. are inventors on a patent application (PCT/US2013/055862) related to this work that has been filed by the Fred Hutchinson Cancer Research Center and licensed by Juno Therapeutics. M. C V体育ios版. J. and S. R. R. are founders and shareholders of Juno Therapeutics. C. R. is inventor on a patent application (PCT/US2011/062670) that claims anti-ROR1 mAb R11 and has been filed by the National Institutes of Health.
Figures
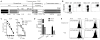





References
-
- Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. - V体育平台登录 - PMC - PubMed
-
- Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–102. - PMC (V体育官网) - PubMed
-
- Nicholson IC, Lenton KA, Little DJ, Decorso T, Lee FT, Scott AM, et al. Construction and characterisation of a functional CD19 specific single chain Fv fragment for immunotherapy of B lineage leukaemia and lymphoma. Mol Immunol. 1997;34:1157–65. - PubMed
-
- Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–84. - V体育官网 - PubMed
Publication types
MeSH terms
- Actions (VSports注册入口)
- Actions (VSports最新版本)
- "VSports注册入口" Actions
- "V体育平台登录" Actions
- Actions (V体育安卓版)
- "VSports注册入口" Actions
- Actions (V体育安卓版)
- "VSports app下载" Actions
- "VSports" Actions
- "VSports注册入口" Actions
- "VSports app下载" Actions
Substances
- VSports - Actions
Grants and funding
LinkOut - more resources
"V体育官网入口" Full Text Sources
Other Literature Sources

