L1CAM promotes enrichment of immunosuppressive T cells in human pancreatic cancer correlating with malignant progression (V体育安卓版)
- PMID: 24746181
- PMCID: PMC5528528
- DOI: 10.1016/j.molonc.2014.03.001
L1CAM promotes enrichment of immunosuppressive T cells in human pancreatic cancer correlating with malignant progression
Abstract
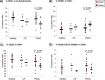
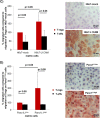
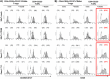
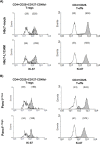
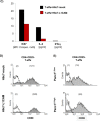
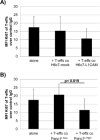
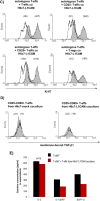
Regulatory T cell (T-reg) enrichment in the tumor microenvironment is regarded as an important mechanism of tumor immune escape. Hence, the presence of T-regs in highly malignant pancreatic ductal adenocarcinoma (PDAC) is correlated with short survival. Likewise, the adhesion molecule L1CAM is upregulated during PDAC progression in the pancreatic ductal epithelium also being associated with poor prognosis. To investigate whether L1CAM contributes to enrichment of T-regs in PDAC, human CD4(+)CD25(+)CD127(-)CD49d(-) T-regs and CD4(+)CD25(-) T-effector cells (T-effs) were isolated by magnetic bead separation from blood of healthy donors VSports手机版. Their phenotype and functional behavior were analyzed in dependence on human premalignant (H6c7) or malignant (Panc1) pancreatic ductal epithelial cells, either exhibiting or lacking L1CAM expression. T cells derived from blood and tumors of PDAC patients were analyzed by flow cytometry and findings were correlated with clinical parameters. Predominantly T-regs but not T-effs showed an increased migration on L1CAM expressing H6c7 and Panc1 cells. Whereas proliferation of T-regs did not change in the presence of L1CAM, T-effs proliferated less, exhibited a decreased CD25 expression and an increased expression of CD69. Moreover, these T-effs exhibited a regulatory phenotype as they inhibited proliferation of autologous T cells. Accordingly, CD4(+)CD25(-)CD69(+) T cells were highly abundant in PDAC tissues compared to blood being associated with nodal invasion and higher grading in PDAC patients. Overall, these data point to an important role of L1CAM in the enrichment of immunosuppressive T cells in particular of a CD4(+)CD25(-)CD69(+)-phenotype in PDAC providing a novel mechanism of tumor immune escape which contributes to tumor progression. .
Keywords: CD171; Immune evasion; Malignant progression; Pancreatic adenocarcinoma; Regulatory T cells; Tumor stroma V体育安卓版. .
Copyright © 2014 Federation of European Biochemical Societies. Published by Elsevier B. V. All rights reserved. V体育ios版.
Figures


References
-
- Ben, Q.-W. , Wang, J.-C. , Liu, J. , Zhu, Y. , Yuan, F. , Yao, W.-Y. , Yuan, Y.-Z. , 2010. Positive expression of L1-CAM is associated with perineural invasion and poor outcome in pancreatic ductal adenocarcinoma. Ann. Surg. Oncol.. 17, 2213–2221. - "V体育ios版" PubMed
-
- Bergmann, F. , Wandschneider, F. , Sipos, B. , Moldenhauer, G. , Schniewind, B. , Welsch, T. , Schirrmacher, P. , Klöppel, G. , Altevogt, P. , Schäfer, H. , Sebens Müerköster, S. , 2010. Elevated L1CAM expression in precursor lesions and primary and metastatic tissues of pancreatic ductal adenocarinoma. Oncol. Rep.. 24, 909–915. - PubMed
-
- Clayton, A. , Al-Taei, S. , Webber, J. , Mason, M.D. , Tabi, Z. , 2011. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J. Immunol.. 187, 676–683. - V体育平台登录 - PubMed
-
- Farrow, B. , Evers, B.M. , 2002. Inflammation and the development of pancreatic cancer. Surg. Oncol.. 10, 153–169. - PubMed
-
- Fogar, P. , Basso, D. , Fadi, E. , Greco, E. , Pantano, G. , Padoan, A. , Bozzato, D. , Facco, M. , Sanzari, M.C. , Teolato, S. , Zambon, C.F. , Navaglia, F. , Semenzato, G. , Pedrazzoli, S. , Plebani, M. , 2011. Pancreatic cancer alters human CD4+ T lymphocyte function: a piece in the immune evasion puzzle. Pancreas. 40, 1131–1137. - "V体育官网入口" PubMed
Publication types
MeSH terms
- "V体育2025版" Actions
- VSports在线直播 - Actions
- VSports app下载 - Actions
- "V体育2025版" Actions
- Actions (V体育平台登录)
"V体育ios版" Substances
- Actions (VSports app下载)
VSports手机版 - LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

