V体育ios版 - Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity
- PMID: 24555999
- PMCID: PMC4340484
- DOI: 10.1136/gutjnl-2013-306271
Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity (V体育2025版)
Abstract
Background: Pancreatic ductal adenocarcinoma (PDA) is characterised by a robust desmoplasia, including the notable accumulation of immunosuppressive cells that shield neoplastic cells from immune detection VSports手机版. Immune evasion may be further enhanced if the malignant cells fail to express high levels of antigens that are sufficiently immunogenic to engender an effector T cell response. .
Objective: To investigate the predominant subsets of immunosuppressive cancer-conditioned myeloid cells that chronicle and shape the progression of pancreas cancer. We show that selective depletion of one subset of myeloid-derived suppressor cells (MDSC) in an autochthonous, genetically engineered mouse model (GEMM) of PDA unmasks the ability of the adaptive immune response to engage and target tumour epithelial cells V体育安卓版. .
Methods: A combination of in vivo and in vitro studies were performed employing a GEMM that faithfully recapitulates the cardinal features of human PDA V体育ios版. The predominant cancer-conditioned myeloid cell subpopulation was specifically targeted in vivo and the biological outcomes determined. .
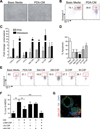
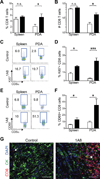
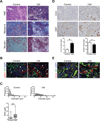
Results: PDA orchestrates the induction of distinct subsets of cancer-associated myeloid cells through the production of factors known to influence myelopoiesis VSports最新版本. These immature myeloid cells inhibit the proliferation and induce apoptosis of activated T cells. Targeted depletion of granulocytic MDSC (Gr-MDSC) in autochthonous PDA increases the intratumoral accumulation of activated CD8 T cells and apoptosis of tumour epithelial cells and also remodels the tumour stroma. .
Conclusions: Neoplastic ductal cells of the pancreas induce distinct myeloid cell subsets that promote tumour cell survival and accumulation V体育平台登录. Targeted depletion of a single myeloid subset, the Gr-MDSC, can unmask an endogenous T cell response, disclosing an unexpected latent immunity and invoking targeting of Gr-MDSC as a potential strategy to exploit for treating this highly lethal disease. .
Keywords: Growth Factors; Immune Response; Pancreatic Cancer. VSports注册入口.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group. bmj. com/group/rights-licensing/permissions. V体育官网入口.
Figures




References
-
- Bayne LJ, Beatty GL, Jhala N, et al. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer cell. 2012;21:822–835. - "V体育安卓版" PMC - PubMed
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 2012;62:10–29. - "V体育官网" PubMed
-
- Hruban R. In: Tumors of the pancreas. Hruban RH, Pitman MB, Klimstra DS, editors. 2007.
Publication types
- "V体育官网入口" Actions
MeSH terms
- Actions (V体育ios版)
- "V体育平台登录" Actions
- VSports注册入口 - Actions
- Actions (VSports注册入口)
- "VSports在线直播" Actions
- "VSports手机版" Actions
- Actions (VSports)
- "V体育安卓版" Actions
- Actions (V体育官网)
- "VSports注册入口" Actions
- "V体育官网入口" Actions
- "VSports" Actions
Substances
- "VSports app下载" Actions
Grants and funding (VSports在线直播)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials (V体育2025版)
