Engineering human peripheral blood stem cell grafts that are depleted of naïve T cells and retain functional pathogen-specific memory T cells
- PMID: 24525279
- PMCID: V体育官网 - PMC3985542
- DOI: 10.1016/j.bbmt.2014.01.032
Engineering human peripheral blood stem cell grafts that are depleted of naïve T cells and retain functional pathogen-specific memory T cells
Abstract
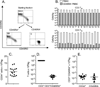
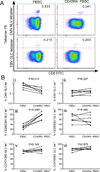
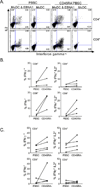
Graft-versus-host disease (GVHD) is a frequent major complication of allogeneic hematopoietic cell transplantation (HCT). Approaches that selectively deplete T cells that cause GVHD from allogeneic stem cell grafts and preserve T cells specific for pathogens may improve HCT outcomes. It has been hypothesized that the majority of T cells that can cause GVHD reside within the naïve T cell (TN) subset, and previous studies performed in mouse models and with human cells in vitro support this hypothesis. As a prelude to translating these findings to the clinic, we developed and evaluated a novel 2-step clinically compliant procedure for manipulating peripheral blood stem cells (PBSC) to remove TN, preserve CD34(+) hematopoietic stem cells, and provide for a fixed dose of memory T cells (TM) that includes T cells with specificity for common opportunistic pathogens encountered after HCT. Our studies demonstrate effective and reproducible performance of the immunomagnetic cell selection procedure for depleting TN. Moreover, after cell processing, the CD45RA-depleted PBSC products are enriched for CD4(+) and CD8(+) TM with a central memory phenotype and contain TM cells that are capable of proliferating and producing effector cytokines in response to opportunistic pathogens VSports手机版. .
Keywords: Graft engineering; Graft-versus-host disease; Immune reconstitution; Memory T cells; Naïve T cells; Selective T cell depletion V体育安卓版. .
Copyright © 2014 American Society for Blood and Marrow Transplantation V体育ios版. Published by Elsevier Inc. All rights reserved. .
Conflict of interest statement
Conflicts of interest: The authors declare no competing financial interests.
V体育官网入口 - Figures





References
-
- Chao NJ. Minors come of age: Minor histocompatibility antigens and graftversus- host disease. Biol Blood Marrow Transplant. 2004;10:215–223. - PubMed
-
- Ferrara JL, Reddy P. Pathophysiology of graft-versus-host disease. Semin Hematol. 2006;43:3–10. - PubMed
-
- Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7:340–352. - PubMed
-
- Bensinger WI, Martin PJ, Storer B, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;344:175–181. - PubMed
V体育平台登录 - Publication types
MeSH terms
- "VSports最新版本" Actions
- Actions (V体育2025版)
- "V体育官网入口" Actions
- V体育官网 - Actions
- V体育官网 - Actions
- "V体育平台登录" Actions
- "VSports注册入口" Actions
- V体育安卓版 - Actions
- VSports - Actions
- VSports最新版本 - Actions
- "VSports在线直播" Actions
Substances
- VSports - Actions
- VSports注册入口 - Actions
- "VSports在线直播" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

