V体育官网 - Photoswitchable nanoparticles for in vivo cancer chemotherapy
- PMID: 24191048
- PMCID: PMC3839756
- DOI: 10.1073/pnas.1315336110
Photoswitchable nanoparticles for in vivo cancer chemotherapy
Abstract
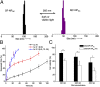
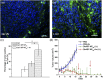
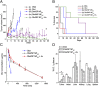
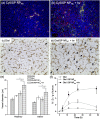
There are many obstacles to effective cancer chemotherapy, including drug penetration and accumulation in tumors and drug systemic toxicity. The penetration of therapies into tumors is limited by the dense tumor matrix and by compression of the tumor vasculature. We have developed spiropyran-based nanoparticles that shrink from 103 to 49 nm upon irradiation at 365 nm. That shrinkage enhanced tissue penetration and drug release. Irradiation of s. c. HT-1080 tumors in nude mice administered i. v. docetaxel-containing nanoparticles was more effective treatment than free docetaxel or encapsulated docetaxel without irradiation. Irradiation at the tumor site also resulted in less systemic toxicity than if the nanoparticles were irradiated before injection, presumably because of less systemically distributed free drug VSports手机版. The enhanced efficacy of nanoparticles in irradiated tumors may have been related to the observed enhanced tumor penetration by nanoparticles and decompression of tumor blood vessels, which may also increase nanoparticle delivery into tumors. .
Keywords: nanomedicine; photoswitching; triggered drug delivery. V体育安卓版.
"VSports在线直播" Conflict of interest statement
The authors declare no conflict of interest.
Figures (V体育ios版)

References
-
- Langer R. Polymer-controlled drug-delivery systems. Acc Chem Res. 1993;26(10):537–542.
-
- Langer R, Folkman J. Polymers for the sustained release of proteins and other macromolecules. Nature. 1976;263(5580):797–800. - PubMed
-
- Gref R, et al. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263(5153):1600–1603. - PubMed
-
- Langer R. Drug delivery and targeting. Nature. 1998;392(6679) Suppl:5–10. - PubMed (VSports手机版)
-
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–6392. - PubMed
Publication types
- Actions (VSports在线直播)
MeSH terms
- "V体育2025版" Actions
- "VSports app下载" Actions
- Actions (V体育官网)
- Actions (V体育ios版)
- "VSports在线直播" Actions
- "V体育2025版" Actions
Substances
- "V体育ios版" Actions
- Actions (V体育ios版)
- VSports手机版 - Actions
Grants and funding
LinkOut - more resources
"V体育2025版" Full Text Sources
Other Literature Sources

