Secondary T cell-T cell synaptic interactions drive the differentiation of protective CD8+ T cells
- PMID: 23475183
- PMCID: PMC3962671
- DOI: 10.1038/ni.2547
"V体育2025版" Secondary T cell-T cell synaptic interactions drive the differentiation of protective CD8+ T cells
Abstract
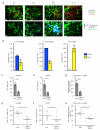
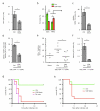
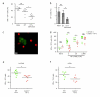
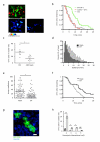
Immunization results in the differentiation of CD8+ T cells, such that they acquire effector abilities and convert into a memory pool VSports手机版. Priming of T cells takes place via an immunological synapse formed with an antigen-presenting cell (APC). By disrupting synaptic stability at different times, we found that the differentiation of CD8+ T cells required cell interactions beyond those made with APCs. We identified a critical differentiation period that required interactions between primed T cells. We found that T cell-T cell synapses had a major role in the generation of protective CD8+ T cell memory. T cell-T cell synapses allowed T cells to polarize critical secretion of interferon-γ (IFN-γ) toward each other. Collective activation and homotypic clustering drove cytokine sharing and acted as regulatory stimuli for T cell differentiation. .
Figures
Comment in
-
T cell priming goes through a new phase.Nat Immunol. 2013 Apr;14(4):311-2. doi: 10.1038/ni.2575. Nat Immunol. 2013. PMID: 23507636 No abstract available.
References
-
- Pipkin ME, et al. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. - "V体育2025版" PMC - PubMed
-
- Prlic M, Williams MA, Bevan MJ. Requirements for CD8 T-cell priming, memory generation and maintenance. Current opinion in immunology. 2007;19:315–319. - "V体育官网" PubMed
-
- Mescher MF, et al. Signals required for programming effector and memory development by CD8+ T cells. Immunological reviews. 2006;211:81–92. - PubMed
-
- Obar JJ, Lefrancois L. Early events governing memory CD8+ T-cell differentiation. International immunology. 2010;22:619–625. - "VSports手机版" PMC - PubMed
Publication types
MeSH terms (VSports注册入口)
- "VSports注册入口" Actions
- Actions (VSports手机版)
- Actions (VSports在线直播)
- "VSports" Actions
- Actions (V体育安卓版)
- VSports app下载 - Actions
- VSports最新版本 - Actions
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials (VSports手机版)
"V体育官网入口" Miscellaneous

