Disrupting galectin-1 interactions with N-glycans suppresses hypoxia-driven angiogenesis and tumorigenesis in Kaposi's sarcoma (V体育安卓版)
- PMID: 23027923
- PMCID: PMC3478924
- DOI: 10.1084/jem.20111665
"V体育官网" Disrupting galectin-1 interactions with N-glycans suppresses hypoxia-driven angiogenesis and tumorigenesis in Kaposi's sarcoma
V体育2025版 - Abstract
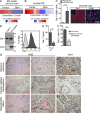
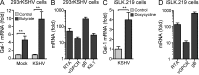
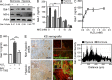
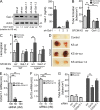
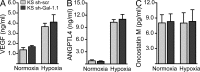
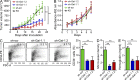
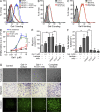
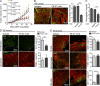
Kaposi's sarcoma (KS), a multifocal vascular neoplasm linked to human herpesvirus-8 (HHV-8/KS-associated herpesvirus [KSHV]) infection, is the most common AIDS-associated malignancy. Clinical management of KS has proven to be challenging because of its prevalence in immunosuppressed patients and its unique vascular and inflammatory nature that is sustained by viral and host-derived paracrine-acting factors primarily released under hypoxic conditions. We show that interactions between the regulatory lectin galectin-1 (Gal-1) and specific target N-glycans link tumor hypoxia to neovascularization as part of the pathogenesis of KS VSports手机版. Expression of Gal-1 is found to be a hallmark of human KS but not other vascular pathologies and is directly induced by both KSHV and hypoxia. Interestingly, hypoxia induced Gal-1 through mechanisms that are independent of hypoxia-inducible factor (HIF) 1α and HIF-2α but involved reactive oxygen species-dependent activation of the transcription factor nuclear factor κB. Targeted disruption of Gal-1-N-glycan interactions eliminated hypoxia-driven angiogenesis and suppressed tumorigenesis in vivo. Therapeutic administration of a Gal-1-specific neutralizing mAb attenuated abnormal angiogenesis and promoted tumor regression in mice bearing established KS tumors. Given the active search for HIF-independent mechanisms that serve to couple tumor hypoxia to pathological angiogenesis, our findings provide novel opportunities not only for treating KS patients but also for understanding and managing a variety of solid tumors. .
Figures

References
-
- Albini A., Morini M., D’Agostini F., Ferrari N., Campelli F., Arena G., Noonan D.M., Pesce C., De Flora S. 2001. Inhibition of angiogenesis-driven Kaposi’s sarcoma tumor growth in nude mice by oral N-acetylcysteine. Cancer Res. 61:8171–8178 - PubMed
-
- Alcendor D.J., Knobel S.M., Desai P., Zhu W.Q., Vigil H.E., Hayward G.S. 2010. KSHV downregulation of galectin-3 in Kaposi’s sarcoma. Glycobiology. 20:521–532 10.1093/glycob/cwp204 - DOI (VSports手机版) - PMC - PubMed
-
- Banh A., Zhang J., Cao H., Bouley D.M., Kwok S., Kong C., Giaccia A.J., Koong A.C., Le Q.T. 2011. Tumor galectin-1 mediates tumor growth and metastasis through regulation of T-cell apoptosis. Cancer Res. 71:4423–4431 10.1158/0008-5472.CAN-10-4157 - DOI (V体育安卓版) - PMC - PubMed
-
- Cedeno-Laurent F., Watanabe R., Teague J.E., Kupper T.S., Clark R.A., Dimitroff C.J. 2012. Galectin-1 inhibits the viability, proliferation, and Th1 cytokine production of nonmalignant T cells in patients with leukemic cutaneous T-cell lymphoma. Blood. 119:3534–3538 10.1182/blood-2011-12-396457 - DOI - PMC - PubMed
Publication types
V体育官网 - MeSH terms
- VSports注册入口 - Actions
- "VSports在线直播" Actions
- V体育官网入口 - Actions
- VSports app下载 - Actions
- "V体育平台登录" Actions
- Actions (V体育官网)
- "VSports注册入口" Actions
- "VSports注册入口" Actions
- Actions (VSports注册入口)
- "V体育平台登录" Actions
- Actions (V体育官网入口)
- V体育安卓版 - Actions
Substances
- VSports app下载 - Actions
- Actions (V体育平台登录)
Grants and funding
"VSports" LinkOut - more resources
Full Text Sources (V体育平台登录)
Other Literature Sources
Medical
Molecular Biology Databases
"VSports在线直播" Research Materials

