"V体育2025版" Population dynamics of naive and memory CD8 T cell responses after antigen stimulations in vivo
- PMID: 22205031
- PMCID: PMC3262935
- DOI: 10.4049/jimmunol.1101579
Population dynamics of naive and memory CD8 T cell responses after antigen stimulations in vivo
Abstract
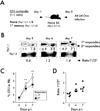
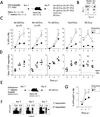
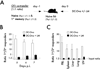
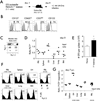
The extent to which the progeny of one primary memory CD8 T cell differs from the progeny of one naive CD8 T cell of the same specificity remains an unresolved question. To explore cell-autonomous functional differences between naive and memory CD8 T cells that are not influenced by differences in the priming environment, an experimental model has been developed in which physiological numbers of both populations of cells were cotransferred into naive hosts before Ag stimulation. Interestingly, naive CD8 T cells undergo greater expansion in numbers than do primary memory CD8 T cells after various infections or immunizations VSports手机版. The intrinsic ability of one naive CD8 T cell to give rise to more effector CD8 T cells than one memory CD8 T cell is independent of the number and quality of primary memory CD8 T cells present in vivo. The sustained proliferation of newly activated naive CD8 T cells contributed to their greater magnitude of expansion. Additionally, longitudinal analyses of primary and secondary CD8 T cell responses revealed that on a per-cell basis naive CD8 T cells generate higher numbers of long-lived memory cells than do primary memory CD8 T cells. This enhanced "memory generation potential" of responding naive CD8 T cells occurred despite the delayed contraction of secondary CD8 T cell responses. Taken together, the data in this study revealed previously unappreciated differences between naive and memory CD8 T cells and will help further define the functional potential for both cell types. .
Conflict of interest statement
The authors have no financial conflict of interest.
Figures




V体育官网入口 - References
-
- Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. - V体育官网 - PubMed
-
- Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–871. - "VSports在线直播" PMC - PubMed
-
- Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. - PubMed
-
- Lefrancois L. Development, trafficking, function of memory T-cell subsets. Immunol Rev. 2006;211:93–103. - PubMed (V体育官网)
Publication types
MeSH terms
- Actions (V体育ios版)
- VSports在线直播 - Actions
- V体育官网 - Actions
Substances
- "VSports注册入口" Actions
"VSports在线直播" Grants and funding
- VSports在线直播 - R01 AI050073/AI/NIAID NIH HHS/United States
- R01 AI083286/AI/NIAID NIH HHS/United States
- R01 AI059752/AI/NIAID NIH HHS/United States
- AI83286/AI/NIAID NIH HHS/United States (VSports最新版本)
- AI59752/AI/NIAID NIH HHS/United States
- R01 AI042767/AI/NIAID NIH HHS/United States
- AI42767/AI/NIAID NIH HHS/United States
- AI50073/AI/NIAID NIH HHS/United States
- VSports最新版本 - R01 AI046653/AI/NIAID NIH HHS/United States
- R37 AI042767/AI/NIAID NIH HHS/United States
- R21 AI042767/AI/NIAID NIH HHS/United States (V体育官网)
- AI46653/AI/NIAID NIH HHS/United States
LinkOut - more resources
"V体育平台登录" Full Text Sources
Research Materials

