VSports手机版 - The impact of pre-existing memory on differentiation of newly recruited naive CD8 T cells
- PMID: 21832161
- PMCID: PMC3169750
- DOI: "VSports在线直播" 10.4049/jimmunol.1100698
"VSports在线直播" The impact of pre-existing memory on differentiation of newly recruited naive CD8 T cells
VSports最新版本 - Abstract
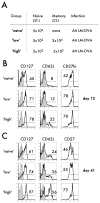
One goal of immunization is to generate memory CD8 T cells of sufficient quality and quantity to confer protection against infection. It has been shown that memory CD8 T cell differentiation in vivo is controlled, at least in part, by the amount and duration of infection, Ag, and inflammatory cytokines present early after the initiation of the response VSports手机版. In this study, we used models of anti-vectorial immunity to investigate the impact of pre-existing immunity on the development and differentiation of vector-induced primary CD8 T cell responses. We showed that existing CD8 T cell memory influences the magnitude of naive CD8 T cell responses. However, the differentiation of newly recruited (either TCR-transgenic or endogenous) primary CD8 T cells into populations with the phenotype (CD62L(hi), CD27(hi), KLRG-1(low)) and function (tissue distribution, Ag-driven proliferation, cytokine production) of long-term memory was facilitated when they were primed in the presence of vector-specific memory CD8 T cells of the same or unrelated specificity. Therefore, these data suggested that the presence of anti-vectorial immunity impacts the rate of differentiation of vector-induced naive CD8 T cells, a notion with important implications for the design of future vaccination strategies. .
Figures





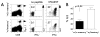
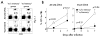
"VSports在线直播" References
-
- Badovinac VP, Harty JT. Programming, demarcating, and manipulating CD8+ T-cell memory. Immunol Rev. 2006;211:67–80. - "V体育官网入口" PubMed
-
- Harty JT, V, Badovinac P. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. - PubMed
-
- Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. - PubMed
-
- Lefrancois L. Development, trafficking, and function of memory T-cell subsets. Immunol Rev. 2006;211:93–103. - PubMed
-
- Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. - PubMed
Publication types
- "VSports在线直播" Actions
V体育2025版 - MeSH terms
- "V体育2025版" Actions
- "V体育ios版" Actions
- Actions (VSports app下载)
- VSports - Actions
Substances
- "VSports手机版" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

