Systemic delivery of NEMO binding domain/IKKγ inhibitory peptide to young mdx mice improves dystrophic skeletal muscle histopathology
- PMID: 21624467
- PMCID: V体育官网 - PMC3145633
- DOI: 10.1016/j.nbd.2011.05.008
Systemic delivery of NEMO binding domain/IKKγ inhibitory peptide to young mdx mice improves dystrophic skeletal muscle histopathology
Abstract
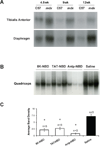
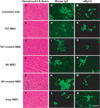
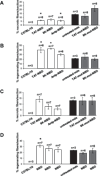
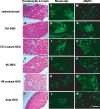
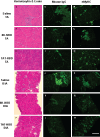
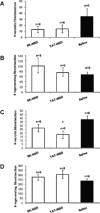
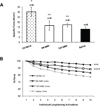
The activation of nuclear factor κB (NF-κB) contributes to muscle degeneration that results from dystrophin deficiency in human Duchenne muscular dystrophy (DMD) and in the mdx mouse VSports手机版. In dystrophic muscle, NF-κB participates in inflammation and failure of muscle regeneration. Peptides containing the NF-κB Essential Modulator (NEMO) binding domain (NBD) disrupt the IκB kinase complex, thus blocking NF-κB activation. The NBD peptide, which is linked to a protein transduction domain to achieve in vivo peptide delivery to muscle tissue, was systemically delivered to mdx mice for 4 or 7 weeks to study NF-κB activation, histological changes in hind limb and diaphragm muscle and ex vivo function of diaphragm muscle. Decreased NF-κB activation, decreased necrosis and increased regeneration were observed in hind limb and diaphragm muscle in mdx mice treated systemically with NBD peptide, as compared to control mdx mice. NBD peptide treatment resulted in improved generation of specific force and greater resistance to lengthening activations in diaphragm muscle ex vivo. Together these data support the potential of NBD peptides for the treatment of DMD by modulating dystrophic pathways in muscle that are downstream of dystrophin deficiency. .
Published by Elsevier Inc.
Figures

References
-
- Acharyya S, Villalta SA, Bakkar N, Bupha-Intr T, Janssen PML, Carathers M, et al. Interplay of IKK/NF-kappa B signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Invest. 2007;117:889–901. - "VSports最新版本" PMC - PubMed
-
- Di MP, Ianaro A, Ghosh S. Amelioration of acute inflammation by systemic administration of a cell-permeable peptide inhibitor of NF-kappaB activation. Arthritis Rheum. 2005;52:951–958. - PubMed
-
- Disatnik MH, Dhawan J, Yu Y, Beal MF, Whirl MM, Franco AA, et al. Evidence of oxidative stress in mdx mouse muscle: studies of the pre-necrotic state. J Neurol Sci. 1998;161:77–84. - PubMed
"V体育官网入口" Publication types
MeSH terms (V体育平台登录)
- "VSports手机版" Actions
- "V体育安卓版" Actions
- Actions (VSports app下载)
- VSports在线直播 - Actions
- Actions (V体育2025版)
- "VSports在线直播" Actions
- Actions (VSports最新版本)
- "V体育安卓版" Actions
- Actions (VSports在线直播)
- "V体育2025版" Actions
- Actions (V体育ios版)
- Actions (V体育2025版)
- "V体育官网" Actions
- Actions (V体育平台登录)
- V体育ios版 - Actions
- "VSports" Actions
- Actions (VSports注册入口)
- V体育平台登录 - Actions
- Actions (V体育安卓版)
Substances
- "V体育官网入口" Actions
- Actions (VSports app下载)
- "V体育2025版" Actions
- V体育官网 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
V体育ios版 - Other Literature Sources
Molecular Biology Databases
Miscellaneous (V体育官网入口)

