"VSports注册入口" Dihydrofolate reductase protects endothelial nitric oxide synthase from uncoupling in tetrahydrobiopterin deficiency
- PMID: 21402147
- PMCID: PMC3121954
- DOI: 10.1016/j.freeradbiomed.2011.03.010
Dihydrofolate reductase protects endothelial nitric oxide synthase from uncoupling in tetrahydrobiopterin deficiency
VSports app下载 - Abstract
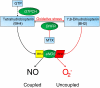
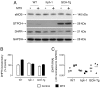
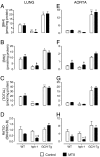
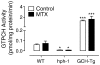
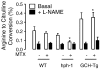
Tetrahydrobiopterin (BH4) is a required cofactor for the synthesis of NO by endothelial nitric oxide synthase (eNOS), and endothelial BH4 bioavailability is a critical factor in regulating the balance between NO and superoxide production (eNOS coupling) VSports手机版. Biosynthesis of BH4 is determined by the activity of GTP-cyclohydrolase I (GTPCH). However, BH4 levels may also be influenced by oxidation, forming 7,8-dihydrobiopterin (BH2), which promotes eNOS uncoupling. Conversely, dihydrofolate reductase (DHFR) can regenerate BH4 from BH2, but whether DHFR is functionally important in maintaining eNOS coupling remains unclear. To investigate the mechanism by which DHFR might regulate eNOS coupling in vivo, we treated wild-type, BH4-deficient (hph-1), and GTPCH-overexpressing (GCH-Tg) mice with methotrexate (MTX), to inhibit BH4 recycling by DHFR. MTX treatment resulted in a striking elevation in BH2 and a decreased BH4:BH2 ratio in the aortas of wild-type mice. These effects were magnified in hph-1 but diminished in GCH-Tg mice. Attenuated eNOS activity was observed in MTX-treated hph-1 but not wild-type or GCH-Tg mouse lung, suggesting that inhibition of DHFR in BH4-deficient states leads to eNOS uncoupling. Taken together, these data reveal a key role for DHFR in regulating the BH4 vs BH2 ratio and eNOS coupling under conditions of low total biopterin availability in vivo. .
Copyright © 2011 Elsevier Inc. All rights reserved V体育安卓版. .
Figures
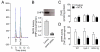
"V体育2025版" References
-
- Hurshman A.R., Krebs C., Edmondson D.E., Huynh B.H., Marletta M.A. Formation of a pterin radical in the reaction of the heme domain of inducible nitric oxide synthase with oxygen. Biochemistry. 1999;38:15689–15696. - PubMed
-
- Schmidt P.P., Lange R., Gorren A.C., Werner E.R., Mayer B., Andersson K.K. Formation of a protonated trihydrobiopterin radical cation in the first reaction cycle of neuronal and endothelial nitric oxide synthase detected by electron paramagnetic resonance spectroscopy. J. Biol. Inorg. Chem. 2001;6:151–158. - PubMed
Publication types
MeSH terms
- "V体育安卓版" Actions
- "V体育官网" Actions
- Actions (V体育官网)
- Actions (VSports手机版)
- "VSports手机版" Actions
- "VSports注册入口" Actions
- V体育官网入口 - Actions
- "V体育安卓版" Actions
- "VSports在线直播" Actions
- "VSports最新版本" Actions
- "V体育安卓版" Actions
- Actions (VSports在线直播)
Substances
- "VSports手机版" Actions
- "VSports手机版" Actions
- VSports在线直播 - Actions
- "V体育官网" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

