Abnormally expressed ER stress response chaperone Gp96 in CD favours adherent-invasive Escherichia coli invasion
- PMID: 20587550
- PMCID: PMC2976078
- DOI: 10.1136/gut.2010.207456
Abnormally expressed ER stress response chaperone Gp96 in CD favours adherent-invasive Escherichia coli invasion (V体育平台登录)
Abstract
Background and aims: Crohn's disease (CD) ileal lesions are colonised by pathogenic adherent-invasive Escherichia coli (AIEC) producing outer membrane vesicles (OMVs) that contribute to the bacterial invasion process VSports手机版. In addition, increased expression of endoplasmic reticulum (ER)-localised stress response proteins, due to ER stress, is observed in patients with CD. The expression of the ER-localised stress response protein Gp96 in patients with CD and its biological role with regards to the ability of AIEC to invade intestinal epithelial cells were analysed. .
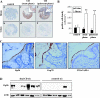
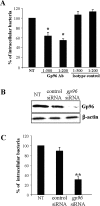
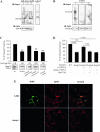
Methods and results: Immunohistochemistry on tissue arrays showed that, together with CEACAM6 (carcinoembryonic antigen-related cell adhesion molecule 6) or the ER stress protein Grp78, Gp96 is also strongly expressed at the apical plasma membrane of the ileal epithelial cells of 50% of patients with CD. Invasion experiments in the presence of antibodies raised against Gp96, or after transfection of Intestine-407 cells with gp96 small interfering RNA (siRNA), indicated that Gp96 is essential to promote AIEC LF82 invasion, allowing, via the recognition of the outer membrane protein OmpA, OMVs to fuse with intestinal epithelial cells. V体育安卓版.
Conclusions: Gp96 is overexpressed on the apical surface of ileal epithelial cells in patients with CD and acts as a host cell receptor for OMVs, promoting AIEC invasion V体育ios版. From the results shown here, it is speculated that AIEC could take advantage of the abnormal expression of Gp96 in patients with CD to invade the ileal mucosa. .
Conflict of interest statement
VSports - Figures

"V体育ios版" References
-
- Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest 2007;117:514–21 - VSports注册入口 - PMC - PubMed
-
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007;448:427–34 - PubMed
-
- Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology 2008;134:577–94 - PubMed (V体育2025版)
-
- Baumgart M, Dogan B, Rishniw M, et al. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn's disease involving the ileum. ISME J 2007;1:403–18 - PubMed
Publication types
MeSH terms
- Actions (V体育2025版)
- Actions (V体育官网入口)
- "VSports在线直播" Actions
- "V体育ios版" Actions
- "V体育安卓版" Actions
- V体育官网入口 - Actions
- VSports app下载 - Actions
- Actions (VSports app下载)
- V体育ios版 - Actions
- "VSports在线直播" Actions
- "VSports在线直播" Actions
Substances
- VSports最新版本 - Actions
- "V体育2025版" Actions
- VSports注册入口 - Actions
- "VSports" Actions
LinkOut - more resources
V体育ios版 - Full Text Sources
Other Literature Sources
Medical
Miscellaneous
