Analysis of microRNA transcriptome by deep sequencing of small RNA libraries of peripheral blood
- PMID: 20459673
- PMCID: PMC2885365
- DOI: 10.1186/1471-2164-11-288
Analysis of microRNA transcriptome by deep sequencing of small RNA libraries of peripheral blood
Abstract (V体育平台登录)
Background: MicroRNAs are a class of small non-coding RNAs that regulate mRNA expression at the post - transcriptional level and thereby many fundamental biological processes. A number of methods, such as multiplex polymerase chain reaction, microarrays have been developed for profiling levels of known miRNAs VSports手机版. These methods lack the ability to identify novel miRNAs and accurately determine expression at a range of concentrations. Deep or massively parallel sequencing methods are providing suitable platforms for genome wide transcriptome analysis and have the ability to identify novel transcripts. .
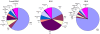
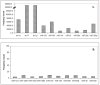
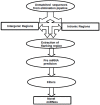
Results: The results of analysis of small RNA sequences obtained by Solexa technology of normal peripheral blood mononuclear cells, tumor cell lines K562 and HL60 are presented. In general K562 cells displayed overall low level of miRNA population and also low levels of DICER. Some of the highly expressed miRNAs in the leukocytes include several members of the let-7 family, miR-21, 103, 185, 191 and 320a. Comparison of the miRNA profiles of normal versus K562 or HL60 cells revealed a specific set of differentially expressed molecules. Correlation of the miRNA with that of mRNA expression profiles, obtained by microarray, revealed a set of target genes showing inverse correlation with miRNA levels. Relative expression levels of individual miRNAs belonging to a cluster were found to be highly variable. Our computational pipeline also predicted a number of novel miRNAs. Some of the predictions were validated by Real-time RT-PCR and or RNase protection assay. Organization of some of the novel miRNAs in human genome suggests that these may also be part of existing clusters or form new clusters V体育安卓版. .
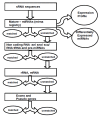
Conclusions: We conclude that about 904 miRNAs are expressed in human leukocytes. Out of these 370 are novel miRNAs. We have identified miRNAs that are differentially regulated in normal PBMC with respect to cancer cells, K562 and HL60 V体育ios版. Our results suggest that post - transcriptional processes may play a significant role in regulating levels of miRNAs in tumor cells. The study also provides a customized automated computation pipeline for miRNA profiling and identification of novel miRNAs; even those that are missed out by other existing pipelines. The Computational Pipeline is available at the website: http://mirna. jnu. ac. in/deep_sequencing/deep_sequencing. html. .
Figures








References
-
- Kulshreshtha R, Davuluri RV, Calin GA, Ivan M. A microRNA component of the hypoxic response. Cell Death Differ. 2008;15:667–671. doi: 10.1038/sj.cdd.4402310. - DOI (V体育平台登录) - PubMed
-
- Spizzo R, Nicoloso MS, Croce CM, Calin GA. SnapShot: MicroRNAs in Cancer. Cell. 2009;137:586–586. doi: 10.1016/j.cell.2009.04.040. e1. - "VSports" DOI - PubMed
Publication types
MeSH terms
- "VSports最新版本" Actions
- "V体育ios版" Actions
Substances
- Actions (V体育平台登录)
"VSports在线直播" Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

