Autophagy, not apoptosis, is essential for midgut cell death in Drosophila
- PMID: 19818615
- PMCID: VSports在线直播 - PMC2783269
- DOI: 10.1016/j.cub.2009.08.042
"V体育官网" Autophagy, not apoptosis, is essential for midgut cell death in Drosophila
Abstract
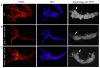
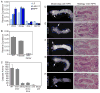
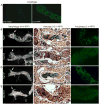
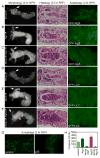
Most developmentally programmed cell death in metazoans is mediated by caspases. During Drosophila metamorphosis, obsolete tissues, including the midgut and salivary glands, are removed by programmed cell death [1]. The initiator caspase Dronc and its activator Ark are required for the death of salivary glands, but not for midgut removal [2, 3]. In addition to caspases, complete removal of salivary glands requires autophagy [4]. However, the contribution of autophagy to midgut cell death has not been explored. Examination of combined mutants of the main initiator and effector caspases revealed that the canonical apoptotic pathway is not required for midgut cell death. Further analyses revealed that the caspase Decay is responsible for most of the caspase activity in dying midguts, yet inhibition of this activity has no effect on midgut removal. By contrast, midgut degradation was severely delayed by inhibition of autophagy, and this occurred without a decrease in caspase activity. Surprisingly, the combined inhibition of caspases and autophagy did not result in an additional delay in midgut removal. Together, our results indicate that autophagy, not caspases, is essential for midgut programmed cell death, providing the first in vivo evidence of caspase-independent programmed cell death that requires autophagy despite the presence of high caspase activity VSports手机版. .
Figures
References
-
- Baehrecke EH. Steroid regulation of programmed cell death during Drosophila development. Cell Death Differ. 2000;7:1057–1062. - PubMed
-
- Daish TJ, Mills K, Kumar S. Drosophila caspase DRONC is required for specific developmental cell death pathways and stress-induced apoptosis. Dev Cell. 2004;7:909–15. - PubMed
-
- Mills K, Daish T, Harvey KF, Pfleger CM, Hariharan IK, Kumar S. The Drosophila melanogaster Apaf-1 homologue ARK is required for most, but not all, programmed cell death. J Cell Biol. 2006;172:809–815. - "VSports在线直播" PMC - PubMed
-
- Chew SK, Akdemir F, Chen P, Lu WJ, Mills K, Daish T, Kumar S, Rodriguez A, Abrams JM. The apical caspase, dronc, governs programmed and unprogrammed cell death in Drosophila. Dev Cell. 2004;7:897–907. - PubMed
Publication types
- VSports最新版本 - Actions
MeSH terms
- Actions (V体育2025版)
- "VSports注册入口" Actions
- "VSports app下载" Actions
- VSports app下载 - Actions
- Actions (V体育官网入口)
- VSports手机版 - Actions
- Actions (VSports最新版本)
Substances
Grants and funding
LinkOut - more resources (VSports)
Full Text Sources
Molecular Biology Databases
Research Materials

