Multiple components contribute to ability of saliva to inhibit influenza viruses
- PMID: 19121065
- PMCID: PMC2848456 (V体育官网入口)
- DOI: 10.1111/j.1399-302X.2008.00468.x
Multiple components contribute to ability of saliva to inhibit influenza viruses
Abstract
Introduction: Saliva is a potentially important barrier against respiratory viral infection but its mechanism of action is not well studied. VSports手机版.
Methods: We tested the antiviral activities of whole saliva, specific salivary gland secretions, and purified salivary proteins against strains of influenza A virus (IAV) in vitro. V体育安卓版.
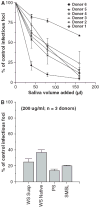
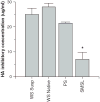
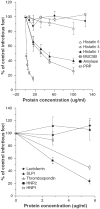
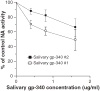
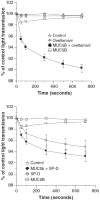
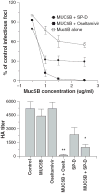
Results: Whole saliva or parotid or submandibular/sublingual secretions from healthy donors inhibited IAV based on hemagglutination inhibition and neutralization assays. This differs from human immunodeficiency virus (HIV), for which only submandibular/sublingual secretions are reported to be inhibitory. Among purified salivary proteins, MUC5B, scavenger receptor cysteine-rich glycoprotein 340 (salivary gp-340), histatins, and human neutrophil defensins (HNPs) inhibited IAV at the concentrations present in whole saliva. In contrast, some abundant salivary proteins (acidic proline-rich proteins and amylase) had no activity, nor did several other less abundant salivary proteins with known activity against HIV (e V体育ios版. g. thrombospondin or serum leukocyte protease inhibitor). Whole saliva and MUC5B did not inhibit neuraminidase activity of IAV and viral neutralizing and aggregating activity of MUC5B was potentiated by the neuraminidase inhibitor oseltamivir. Hence, MUC5B inhibits IAV by presenting a sialic acid ligand for the viral hemagglutinin. The mechanism of action of histatins requires further study. .
Conclusions: These findings indicate that saliva represents an important initial barrier to IAV infection and underline the complexity of host defense activity of oral secretions VSports最新版本. Of interest, antiviral activity of saliva against IAV and HIV differs in terms of specific glandular secretions and proteins that are inhibitory. .
V体育ios版 - Conflict of interest statement
The authors have no conflict of interest related to research presented in this manuscript.
"V体育官网" Figures
"V体育2025版" References
-
- Archibald DW, Cole GA. In vitro inhibition of HIV-1 infectivity by human salivas. AIDS Res Hum Retroviruses. 1990;6:1425–1432. - PubMed
-
- Bolscher JG, Nazmi K, Ran LJ, et al. Inhibition of HIV-1 IIIB and clinical isolates by human parotid, submandibular, sublingual and palatine saliva. Eur J Oral Sci. 2002;110:149–156. - PubMed (VSports)
-
- Crombie R, Kawasaki K, Hojo K, Laurence J. Peptides derived from salivary thrombospondin-1 replicate its anti-HIV effect: potential role in microbicide development. J Acquir Immune Defic Syndr. 2001;27:91–93. - PubMed (V体育ios版)
Publication types
- "V体育官网入口" Actions
MeSH terms
- "VSports最新版本" Actions
- Actions (V体育官网入口)
- "VSports" Actions
- Actions (V体育平台登录)
- "VSports" Actions
- "V体育2025版" Actions
- Actions (V体育官网)
- V体育平台登录 - Actions
- Actions (VSports)
- Actions (V体育ios版)
- "V体育2025版" Actions
- "V体育2025版" Actions
- "V体育官网" Actions
- Actions (V体育2025版)
- "V体育官网入口" Actions
- "V体育平台登录" Actions
Substances
- Actions (V体育安卓版)
- V体育官网入口 - Actions
- Actions (VSports在线直播)
- "VSports手机版" Actions
- Actions (V体育2025版)
Grants and funding
- U01 DE017788/DE/NIDCR NIH HHS/United States
- F31 DE005672/DE/NIDCR NIH HHS/United States
- R01 HL069031/HL/NHLBI NIH HHS/United States
- DE1495/DE/NIDCR NIH HHS/United States
- R01 DE005672/DE/NIDCR NIH HHS/United States
- DE07652/DE/NIDCR NIH HHS/United States (V体育2025版)
- R03 DE016699/DE/NIDCR NIH HHS/United States
- R37 DE005672/DE/NIDCR NIH HHS/United States
- "V体育ios版" R01 DE007652/DE/NIDCR NIH HHS/United States
- "VSports在线直播" DE16699/DE/NIDCR NIH HHS/United States
- "VSports" DE05672/DE/NIDCR NIH HHS/United States
- HL69031/HL/NHLBI NIH HHS/United States (V体育2025版)
LinkOut - more resources
Full Text Sources
Other Literature Sources

