HMGB1 regulates RANKL-induced osteoclastogenesis in a manner dependent on RAGE
- PMID: 18302500
- PMCID: PMC2679382
- DOI: 10.1359/jbmr.080234
HMGB1 regulates RANKL-induced osteoclastogenesis in a manner dependent on RAGE
Abstract
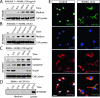
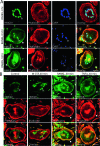
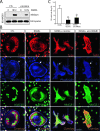
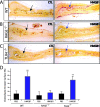
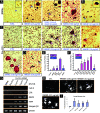
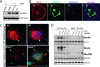
High-mobility group box 1 (HMGB1), a nonhistone nuclear protein, is released by macrophages into the extracellular milieu consequent to cellular activation. Extracellular HMGB1 has properties of a pro-inflammatory cytokine through its interaction with receptor for advanced glycation endproducts (RAGE) and/or toll-like receptors (TLR2 and TLR4) VSports手机版. Although HMGB1 is highly expressed in macrophages and differentiating osteoclasts, its role in osteoclastogenesis remains largely unknown. In this report, we present evidence for a function of HMGB1 in this event. HMGB1 is released from macrophages in response to RANKL stimulation and is required for RANKL-induced osteoclastogenesis in vitro and in vivo. In addition, HMGB1, like other osteoclastogenic cytokines (e. g. , TNFalpha), enhances RANKL-induced osteoclastogenesis in vivo and in vitro at subthreshold concentrations of RANKL, which alone would be insufficient. The role of HMGB1 in osteoclastogenesis is mediated, in large part, by its interaction with RAGE, an immunoglobin domain containing family receptor that plays an important role in osteoclast terminal differentiation and activation. HMGB1-RAGE signaling seems to be important in regulating actin cytoskeleton reorganization, thereby participating in RANKL-induced and integrin-dependent osteoclastogenesis. Taken together, these observations show a novel function of HMGB1 in osteoclastogenesis and provide a new link between inflammatory mechanisms and bone resorption. .
Figures


References
-
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. - PubMed
-
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597–3602. - PMC - PubMed
-
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. - PubMed
-
- Teitelbaum SL. Osteoclasts, integrins, and osteoporosis. J Bone Miner Metab. 2000;18:344–349. - "V体育平台登录" PubMed
Publication types
MeSH terms
- VSports手机版 - Actions
- V体育ios版 - Actions
- V体育官网 - Actions
- V体育平台登录 - Actions
- "V体育官网" Actions
- "VSports app下载" Actions
- Actions (V体育2025版)
- Actions (VSports最新版本)
Substances
- V体育平台登录 - Actions
- "V体育ios版" Actions
Grants and funding
LinkOut - more resources (V体育2025版)
Full Text Sources
V体育2025版 - Other Literature Sources

