Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury
- PMID: 18187572
- PMCID: PMC2312361
- DOI: 10.2353/ajpath.2008.070876
Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury
Abstract
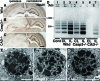
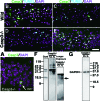
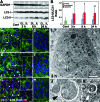
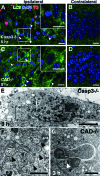
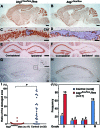
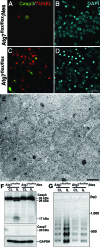
Neonatal hypoxic/ischemic (H/I) brain injury causes neurological impairment, including cognitive and motor dysfunction as well as seizures. However, the molecular mechanisms regulating neuron death after H/I injury are poorly defined and remain controversial. Here we show that Atg7, a gene essential for autophagy induction, is a critical mediator of H/I-induced neuron death. Neonatal mice subjected to H/I injury show dramatically increased autophagosome formation and extensive hippocampal neuron death that is regulated by both caspase-3-dependent and -independent execution VSports手机版. Mice deficient in Atg7 show nearly complete protection from both H/I-induced caspase-3 activation and neuron death indicating that Atg7 is critically positioned upstream of multiple neuronal death executioner pathways. Adult H/I brain injury also produces a significant increase in autophagy, but unlike neonatal H/I, neuron death is almost exclusively caspase-3-independent. These data suggest that autophagy plays an essential role in triggering neuronal death execution after H/I injury and Atg7 represents an attractive therapeutic target for minimizing the neurological deficits associated with H/I brain injury. .
Figures




Comment in
-
Eaten alive: autophagy and neuronal cell death after hypoxia-ischemia.Am J Pathol. 2008 Feb;172(2):284-7. doi: 10.2353/ajpath.2008.071064. Epub 2008 Jan 17. Am J Pathol. 2008. PMID: 18202199 Free PMC article. Review. No abstract available.
References
-
- du Plessis AJ, Volpe JJ. Perinatal brain injury in the preterm and term newborn. Curr Opin Neurol. 2002;15:151–157. - V体育安卓版 - PubMed
-
- Hamrick SE, Ferriero DM. The injury response in the term newborn brain: can we neuroprotect? Curr Opin Neurol. 2003;16:147–154. - PubMed
-
- Ashwal S, Pearce WJ. Animal models of neonatal stroke. Curr Opin Pediatr. 2001;13:506–516. - "VSports app下载" PubMed
-
- Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351:1985–1995. - PubMed
-
- Hagberg H, Bona E, Gilland E, Puka-Sundvall M. Hypoxia-ischaemia model in the 7-day-old rat: possibilities and shortcomings. Acta Paediatr Suppl. 1997;422:85–88. - PubMed
Publication types
- Actions (VSports)
MeSH terms
- V体育官网 - Actions
- V体育官网 - Actions
- Actions (V体育安卓版)
- "VSports在线直播" Actions
- "V体育ios版" Actions
- Actions (VSports)
- V体育官网入口 - Actions
- VSports最新版本 - Actions
- "VSports app下载" Actions
- "V体育2025版" Actions
- Actions (VSports最新版本)
- VSports最新版本 - Actions
Substances
- V体育ios版 - Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources (V体育官网)
Molecular Biology Databases
Research Materials

