A Foxo/Notch pathway controls myogenic differentiation and fiber type specification
- PMID: 17717603
- PMCID: PMC1950461
- DOI: 10.1172/JCI32054
A Foxo/Notch pathway controls myogenic differentiation and fiber type specification
Abstract
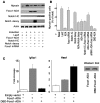
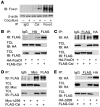
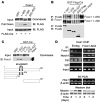
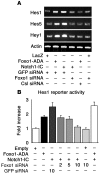
Forkhead box O (Foxo) transcription factors govern metabolism and cellular differentiation. Unlike Foxo-dependent metabolic pathways and target genes, the mechanisms by which these proteins regulate differentiation have not been explored. Activation of Notch signaling mimics the effects of Foxo gain of function on cellular differentiation. Using muscle differentiation as a model system, we show that Foxo physically and functionally interacts with Notch by promoting corepressor clearance from the Notch effector Csl, leading to activation of Notch target genes. Inhibition of myoblast differentiation by constitutively active Foxo1 is partly rescued by inhibition of Notch signaling while Foxo1 loss of function precludes Notch inhibition of myogenesis and increases myogenic determination gene (MyoD) expression. Accordingly, conditional Foxo1 ablation in skeletal muscle results in increased formation of MyoD-containing (fast-twitch) muscle fibers and altered fiber type distribution at the expense of myogenin-containing (slow-twitch) fibers VSports手机版. Notch/Foxo1 cooperation may integrate environmental cues through Notch with metabolic cues through Foxo1 to regulate progenitor cell maintenance and differentiation. .
V体育官网入口 - Figures


"V体育官网入口" References
-
- Accili D., Arden K.C. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/S0092-8674(04)00452-0. - "V体育平台登录" DOI - PubMed
Publication types
- "V体育ios版" Actions
MeSH terms
- Actions (V体育官网入口)
- Actions (V体育官网入口)
- Actions (VSports在线直播)
- "V体育ios版" Actions
- Actions (VSports在线直播)
- VSports - Actions
- V体育官网入口 - Actions
- Actions (VSports)
- V体育ios版 - Actions
"VSports注册入口" Substances
- VSports在线直播 - Actions
- V体育ios版 - Actions
- "V体育官网" Actions
- V体育ios版 - Actions
- "V体育2025版" Actions
Grants and funding
LinkOut - more resources (V体育平台登录)
VSports在线直播 - Full Text Sources
Other Literature Sources
Molecular Biology Databases
VSports app下载 - Research Materials
Miscellaneous

