PhoPQ-mediated regulation produces a more robust permeability barrier in the outer membrane of Salmonella enterica serovar typhimurium
- PMID: 17693506
- PMCID: VSports注册入口 - PMC2168427
- DOI: 10.1128/JB.00973-07
PhoPQ-mediated regulation produces a more robust permeability barrier in the outer membrane of Salmonella enterica serovar typhimurium
Abstract
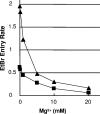
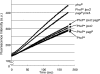
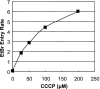
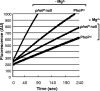
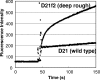
The PhoPQ two-component system of Salmonella enterica serovar Typhimurium produces a remodeling of the lipid A domain of the lipopolysaccharide, including the PagP-catalyzed addition of palmitoyl residue, the PmrAB-regulated addition of the cationic sugar 4-aminoarabinose and phosphoethanolamine, and the LpxO-catalyzed addition of a 2-OH group onto one of the fatty acids. By using the diffusion rates of the dyes ethidium, Nile red, and eosin Y across the outer membrane, as well as the susceptibility of cells to large, lipophilic agents, we evaluated the function of this membrane as a permeability barrier. We found that the remodeling process in PhoP-constitutive strains produces an outer membrane that serves as a very effective permeability barrier in an environment that is poor in divalent cations or that contains cationic peptides, whereas its absence in phoP null mutants produces an outer membrane severely compromised in its barrier function under these conditions. Removing combinations of the lipid A-remodeling functions from a PhoP-constitutive strain showed that the known modification reactions explain a major part of the PhoPQ-regulated changes in permeability. We believe that the increased barrier property of the remodeled bilayer is important in making the pathogen more resistant to the stresses that it encounters in the host, including attack by the cationic antimicrobial peptides. On the other hand, drug-induced killing assays suggest that the outer membrane containing unmodified lipid A may serve as a better barrier in the presence of high concentrations (e. g VSports手机版. , 5 mM) of Mg(2+). .
"V体育平台登录" Figures





References
-
- Adams, M. H. 1959. Bacteriophages, p. 445-447. Wiley Interscience, New York, NY.
-
- Ames, B. N., F. D. Lee, and W. E. Durston. 1973. An improved bacterial test system for the detection and classification of mutagens and carcinogens. Proc. Natl. Acad. Sci. USA 70:782-786. - "V体育官网" PMC - PubMed
-
- Bader, M. W., W. W. Navarre, W. Shiau, H. Nikaido, J. G. Frye, M. McClelland, F. C. Fang, and S. I. Miller. 2003. Regulation of Salmonella typhimurium virulence gene expression by cationic antimicrobial peptides. Mol. Microbiol. 50:219-230. - PubMed
-
- Bader, M. W., S. Sanowar, M. E. Daley, A. R. Schneider, U. Cho, W. Xu, R. E. Klevit, H. Le Moual, and S. I. Miller. 2005. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell 122:461-472. - PubMed
Publication types
MeSH terms
- Actions (V体育平台登录)
- "VSports app下载" Actions
- "VSports注册入口" Actions
- "VSports最新版本" Actions
- Actions (VSports app下载)
- Actions (VSports手机版)
- "V体育官网入口" Actions
- "V体育官网" Actions
- Actions (V体育官网)
- "V体育2025版" Actions
- Actions (V体育ios版)
V体育ios版 - Substances
- V体育官网 - Actions
- Actions (VSports手机版)
- Actions (V体育安卓版)
- Actions (VSports最新版本)
- V体育ios版 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
"V体育安卓版" Other Literature Sources

