Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase
- PMID: 15941833
- PMCID: PMC1157015
- DOI: 10.1073/pnas.0409594102 (VSports手机版)
VSports最新版本 - Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase
Abstract
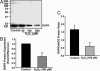
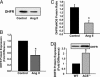
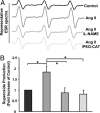
Recent studies demonstrate that oxidative inactivation of tetrahydrobiopterin (H4B) may cause uncoupling of endothelial nitric oxide synthase (eNOS) to produce superoxide (O2*-). H4B was found recyclable from its oxidized form by dihydrofolate reductase (DHFR) in several cell types. Functionality of the endothelial DHFR, however, remains completely unknown. Here we present findings that specific inhibition of endothelial DHFR by RNA interference markedly reduced endothelial H4B and nitric oxide (NO. ) bioavailability VSports手机版. Furthermore, angiotensin II (100 nmol/liter for 24 h) caused a H4B deficiency that was mediated by H2O2-dependent down-regulation of DHFR. This response was associated with a significant increase in endothelial O2*- production, which was abolished by eNOS inhibitor N-nitro-L-arginine-methyl ester or H2O2 scavenger polyethylene glycol-conjugated catalase, strongly suggesting H2O2-dependent eNOS uncoupling. Rapid and transient activation of endothelial NAD(P)H oxidases was responsible for the initial burst production of O2* (Rac1 inhibitor NSC 23766 but not an N-nitro-L-arginine-methyl ester-attenuated ESR O2*- signal at 30 min) in response to angiotensin II, preceding a second peak in O2*- production at 24 h that predominantly depended on uncoupled eNOS. Overexpression of DHFR restored NO. production and diminished eNOS production of O2*- in angiotensin II-stimulated cells. In conclusion, these data represent evidence that DHFR is critical for H4B and NO. bioavailability in the endothelium. Endothelial NAD(P)H oxidase-derived H2O2 down-regulates DHFR expression in response to angiotensin II, resulting in H4B deficiency and uncoupling of eNOS. This signaling cascade may represent a universal mechanism underlying eNOS dysfunction under pathophysiological conditions associated with oxidant stress. .
Figures


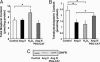
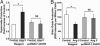
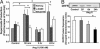
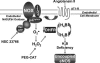
References
-
- Cai, H. (2005) Circ. Res. 96, 818–822. - PubMed (V体育平台登录)
-
- Cai, H., Griendling, K. K. & Harrison, D. G. (2003) Trends Pharmacol. Sci. 24, 471–478. - PubMed
-
- Cai, H. & Harrison, D. G. (2000) Circ. Res. 87, 840–844. - PubMed (VSports最新版本)
-
- Lassegue, B. & Griendling, K. K. (2004) Am. J. Hypertens. 17, 852–860. - PubMed
-
- Harrison, D. G., Cai, H., Landmesser, U. & Griendling, K. K. (2003) J. Renin Angiotensin Aldosterone Syst. 4, 51–61. - PubMed
Publication types
- Actions (VSports app下载)
VSports在线直播 - MeSH terms
- V体育安卓版 - Actions
- VSports最新版本 - Actions
- VSports app下载 - Actions
- "V体育2025版" Actions
- "VSports在线直播" Actions
- "VSports注册入口" Actions
- V体育平台登录 - Actions
Substances (V体育平台登录)
- Actions (VSports app下载)
- "VSports注册入口" Actions
- "VSports手机版" Actions
- V体育平台登录 - Actions
Grants and funding
LinkOut - more resources
VSports最新版本 - Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

