Activated liver X receptors stimulate adipocyte differentiation through induction of peroxisome proliferator-activated receptor gamma expression
- PMID: 15060163
- PMCID: PMC381668 (V体育ios版)
- DOI: 10.1128/MCB.24.8.3430-3444.2004
V体育平台登录 - Activated liver X receptors stimulate adipocyte differentiation through induction of peroxisome proliferator-activated receptor gamma expression
Abstract
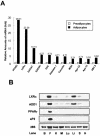
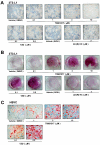
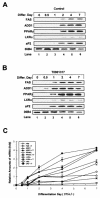
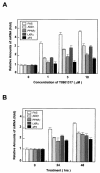
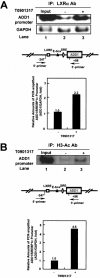
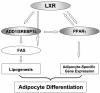
Liver X receptors (LXRs) are nuclear hormone receptors that regulate cholesterol and fatty acid metabolism in liver tissue and in macrophages. Although LXR activation enhances lipogenesis, it is not well understood whether LXRs are involved in adipocyte differentiation. Here, we show that LXR activation stimulated the execution of adipogenesis, as determined by lipid droplet accumulation and adipocyte-specific gene expression in vivo and in vitro. In adipocytes, LXR activation with T0901317 primarily enhanced the expression of lipogenic genes such as the ADD1/SREBP1c and FAS genes and substantially increased the expression of the adipocyte-specific genes encoding PPARgamma (peroxisome proliferator-activated receptor gamma) and aP2. Administration of the LXR agonist T0901317 to lean mice promoted the expression of most lipogenic and adipogenic genes in fat and liver tissues. It is of interest that the PPARgamma gene is a novel target gene of LXR, since the PPARgamma promoter contains the conserved binding site of LXR and was transactivated by the expression of LXRalpha. Moreover, activated LXRalpha exhibited an increase of DNA binding to its target gene promoters, such as ADD1/SREBP1c and PPARgamma, which appeared to be closely associated with hyperacetylation of histone H3 in the promoter regions of those genes. Furthermore, the suppression of LXRalpha by small interfering RNA attenuated adipocyte differentiation. Taken together, these results suggest that LXR plays a role in the execution of adipocyte differentiation by regulation of lipogenesis and adipocyte-specific gene expression. VSports手机版.
Figures



References
-
- Akiyama, T. E., S. Sakai, G. Lambert, C. J. Nicol, K. Matsusue, S. Pimprale, Y. H. Lee, M. Ricote, C. K. Glass, H. B. Brewer, Jr., and F. J. Gonzalez. 2002. Conditional disruption of the peroxisome proliferator-activated receptor gamma gene in mice results in lowered expression of ABCA1, ABCG1, and apoE in macrophages and reduced cholesterol efflux. Mol. Cell. Biol. 22:2607-2619. - PMC - PubMed
-
- Amemiya-Kudo, M., H. Shimano, T. Yoshikawa, N. Yahagi, A. H. Hasty, H. Okazaki, Y. Tamura, F. Shionoiri, Y. Iizuka, K. Ohashi, J. Osuga, K. Harada, T. Gotoda, R. Sato, S. Kimura, S. Ishibashi, and N. Yamada. 2000. Promoter analysis of the mouse sterol regulatory element-binding protein-1c gene. J. Biol. Chem. 275:31078-31085. - PubMed
-
- Barak, Y., M. C. Nelson, E. S. Ong, Y. Z. Jones, P. Ruiz-Lozano, K. R. Chien, A. Koder, and R. M. Evans. 1999. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol. Cell 4:585-595. - PubMed
-
- Cao, G., Y. Liang, C. L. Broderick, B. A. Oldham, T. P. Beyer, R. J. Schmidt, Y. Zhang, K. R. Stayrook, C. Suen, K. A. Otto, A. R. Miller, J. Dai, P. Foxworthy, H. Gao, T. P. Ryan, X. C. Jiang, T. P. Burris, P. I. Eacho, and G. J. Etgen. 2003. Antidiabetic action of a liver X receptor agonist mediated by inhibition of hepatic gluconeogenesis. J. Biol. Chem. 278:1131-1136. - PubMed (V体育平台登录)
-
- Chawla, A., W. A. Boisvert, C. H. Lee, B. A. Laffitte, Y. Barak, S. B. Joseph, D. Liao, L. Nagy, P. A. Edwards, L. K. Curtiss, R. M. Evans, and P. Tontonoz. 2001. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol. Cell 7:161-171. - V体育平台登录 - PubMed
"V体育平台登录" Publication types
VSports app下载 - MeSH terms
- "V体育ios版" Actions
- Actions (V体育2025版)
- Actions (V体育官网)
- V体育安卓版 - Actions
- VSports手机版 - Actions
- Actions (V体育ios版)
- Actions (VSports最新版本)
- V体育官网 - Actions
- "VSports手机版" Actions
- VSports手机版 - Actions
- VSports手机版 - Actions
- "V体育安卓版" Actions
- Actions (VSports app下载)
- "V体育ios版" Actions
- "V体育2025版" Actions
- "VSports注册入口" Actions
- Actions (VSports注册入口)
Substances
- Actions (V体育ios版)
- "VSports注册入口" Actions
- V体育2025版 - Actions
- "VSports最新版本" Actions
- "V体育2025版" Actions
"VSports在线直播" LinkOut - more resources
Full Text Sources
"V体育2025版" Other Literature Sources
Molecular Biology Databases
Research Materials
"VSports在线直播" Miscellaneous
