"V体育官网入口" A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus
- PMID: 11504915
- PMCID: PMC58775
- DOI: 10.1073/pnas.181181198
A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus
Abstract
The Mdm2 oncoprotein promotes cell survival and cell cycle progression by inhibiting the p53 tumor suppressor protein. To regulate p53, Mdm2 must gain nuclear entry, and the mechanism that induces this is now identified. Mitogen-induced activation of phosphatidylinositol 3-kinase (PI3-kinase) and its downstream target, the Akt/PKB serine-threonine kinase, results in phosphorylation of Mdm2 on serine 166 and serine 186. Phosphorylation on these sites is necessary for translocation of Mdm2 from the cytoplasm into the nucleus. Pharmacological blockade of PI3-kinase/Akt signaling or expression of dominant-negative PI3-kinase or Akt inhibits nuclear entry of Mdm2, increases cellular levels of p53, and augments p53 transcriptional activity VSports手机版. Expression of constitutively active Akt promotes nuclear entry of Mdm2, diminishes cellular levels of p53, and decreases p53 transcriptional activity. Mutation of the Akt phosphorylation sites in Mdm2 produces a mutant protein that is unable to enter the nucleus and increases p53 activity. The demonstration that PI3-kinase/Akt signaling affects Mdm2 localization provides insight into how this pathway, which is inappropriately activated in many malignancies, affects the function of p53. .
Figures
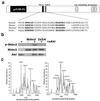
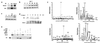
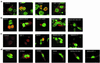
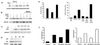
Comment in
-
"V体育官网入口" AKT plays a central role in tumorigenesis.Proc Natl Acad Sci U S A. 2001 Sep 25;98(20):10983-5. doi: 10.1073/pnas.211430998. Proc Natl Acad Sci U S A. 2001. PMID: 11572954 Free PMC article. Review. No abstract available.
V体育2025版 - References
-
- Agarwal M L, Taylor W R, Chernov M V, Chernova O B, Stark G R. J Biol Chem. 1997;273:1–4. - PubMed (V体育官网)
-
- Levine A J. Cell. 1997;88:323–331. - PubMed
-
- Donehower L A, Harvey M, Slagle B L, McArthur M J, Montgomery C A, Jr, Butel J S, Bradley A. Nature (London) 1992;356:215–221. - PubMed
-
- Harvey M, McArthur M J, Montgomery C A, Jr, Butel J S, Bradley A, Donehower L A. Nat Genet. 1993;5:225–229. - PubMed
Publication types
- V体育安卓版 - Actions
- Actions (V体育官网入口)
MeSH terms
- Actions (V体育官网入口)
- "VSports注册入口" Actions
- "V体育官网入口" Actions
- VSports app下载 - Actions
- V体育官网 - Actions
- "V体育安卓版" Actions
- "V体育ios版" Actions
- Actions (V体育安卓版)
- Actions (VSports手机版)
- VSports - Actions
- "VSports手机版" Actions
- "V体育官网入口" Actions
- Actions (VSports app下载)
- "V体育官网入口" Actions
- VSports最新版本 - Actions
- V体育2025版 - Actions
Substances
- Actions (V体育2025版)
- VSports在线直播 - Actions
- "V体育官网" Actions
"VSports在线直播" Grants and funding
LinkOut - more resources
Full Text Sources
"VSports手机版" Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

