V体育官网入口 - Abstract
Tumors may adopt normal physiologic checkpoints for immunomodulation leading to an imbalance between tumor growth and host surveillance. Antibodies targeting the PD-1/PD-L1 checkpoint have shown dynamic and durable tumor regressions, suggesting a rebalancing of the host-tumor interaction. Nivolumab and pembrolizumab are the anti-PD-1 antibodies that are currently the furthest in clinical development, and anti-PD-L1 agents under investigation include MPDL3280A, MEDI4736, and BMS-936559 VSports最新版本. These agents have been used to treat advanced melanoma, non-small cell lung cancer, renal cell carcinoma, bladder cancer and Hodgkin lymphoma, amongst other tumor types. In this article, we review the updated response results for early clinical trials, note recent FDA actions regarding this class of agents, and summarize results across trials looking at PD-L1 status as a predictor of response to anti-PD-1/PD-L1.
Keywords: PD-1, PD-L1, checkpoint blockade, biomarker, FDA
Introduction
Antibodies blocking PD-1 and PD-L1 have demonstrated durable responses in a number of different advanced malignancies. The PD-1/PD-L1 checkpoint is operative in peripheral tissues and serves as a negative regulator of T-cells to help control local inflammatory responses and maintain self-tolerance. PD-1 is expressed on activated T-cells, natural killer cells, and B-cells [1] VSports注册入口. Its two known ligands are PD-L1 (B7-H1) and PD-L2 (B7-DC). PD-L1 is constitutively expressed on a subset of macrophages, but may be rapidly upregulated in a number of different tissue types and by tumors in response to interferon-gamma and other inflammatory mediators [2–4]. PD-L2 is expressed on macrophages and dendritic cells, though it’s impact on surveilling T-cells is not as well understood [5,6]. PD-L2 expression by tumors as a mechanism of immune evasion has also been described. In addition to ligating PD-1, PD-L1 can also bind CD80 on activated T-cells. The observed differences in clinical activity and types of immune-related adverse events between anti-PD-1 and -PD-L1 may be attributable to the interaction between PD-L1 and CD80, as well as a suspected second receptor for PD-L2 [7].
A number of agents targeting both sides of the PD-1/PD-L1 interaction are currently in clinical development, Table 1. This review covers the first landmark trials reported in 2012 that employed these agents to treat multiple different solid tumor types, as well as summarizes the response rates by tumor type from subsequent trials V体育官网入口. Most tumors are thought to display antigens that can be recognized by T-cells, though some are thought to be more “immunogenic” than others, for example, mismatch-repair deficient colorectal carcinomas have a high mutational density and thus a greater likelihood of generating a strongly antigenic mutation, and melanomas display melanocyte-specific antigens that are readily recognized by the immune system. It was anticipated that tumor types such as these would most likely to respond to this therapy, and one of the more exciting developments has been the dramatic clinical responses in patients with less immunogenic tumor types such as non-small cell lung cancer (NSCLC). The remarkable results observed in these trials have resulted in recent FDA approvals for pembrolizumab and nivolumab (both anti-PD-1 antibodies) for the treatment of advanced melanoma in late 2014 and nivolumab for the treatment of non-small cell lung carcinoma in early 2015.
Table 1.
PD-1 and PD-L1 inhibitors in clinical development.
| Target | Biologic agent | Class | Company |
|---|---|---|---|
| PD-1 | AMP-224 | PD-L2 IgG2a fusion protein | Amplimmune/GlaxoSmith Klein |
| AMP-514 (MEDI0680) | PD-L2 fusion protein | Amplimmune/GlaxoSmith Klein | |
| Nivolumab (Opdivo, BMS-936558, MDX1106) | Human IgG4 | Bristol-Meyers Squibb | |
| Pidilizumab (CT-011) | Humanized IgG1k | Cure Tech | |
| Pembrolizumab (MK-3475, (lambrolizumab) | Humanized IgG4 | Merck | |
| PD-L1 | BMS-936559 (MDX1105) | Human IgG4 | Bristol-Meyers Squibb |
| MEDI4736 | Humanized IgG1k | MedImmune/AstraZeneca | |
| MPDL3280A | Human IgG1k | Roche | |
| MSB0010718C | Human IgG1 | Merck |
VSports在线直播 - Early Clinical Development
The first studies demonstrating anti-tumor efficacy with anti-PD-1/PD-L1 in multiple solid tumor types
The first-in-human report of anti-PD-1 in solid tumors included 39 patients with advanced melanoma, NSCLC, renal cell carcinoma (RCC), prostate and colorectal cancer who had received MDX1106 (nivolumab) V体育2025版. Anti-tumor activity was observed, and PD-1 receptor occupancy studies indicated a longer than anticipated half-life for the agent [8]. These findings were pursued in a larger cohort of 296 patients, and objective responses (OR) as defined by RECIST with modifications [9] were observed in 26 of 94 (28%), 14 of 76 (18%) and 9 of 33 (27%) of heavily pre-treated patients with melanoma, NSCLC, and RCC, respectively [10]. An additional subset of patients demonstrated prolonged disease stabilization. OR were not seen in patients with colorectal or prostate cancer. The responses were durable, with approximately 2/3 of responses lasting for at least one year. In general, the safety profile was acceptable (see discussion below on Immune-related adverse events).
A companion report investigated BMS-936559 (anti-PD-L1) in 207 patients in multiple tumor types [11]. OR were observed in 9 of 52 (17%) of patients with melanoma, 2 of 17 (12%) with RCC, 5/49 with NSCLC (10%) and 1 of 17 (67%) with ovarian cancer, and responses, when observed, were also durable VSports. Responses were not seen in patients with pancreatic, gastric, colorectal, or breast cancers. Collectively, these studies supported the further clinical development of PD-1/PD-L1 blockade for the treatment of multiple different types of advanced cancer.
"V体育ios版" Melanoma
In addition to the 94 patients treated with nivolumab described above, the response rates for an additional ~650 advanced melanoma patients treated with other anti-PD-1 agents on early phase trials have been reported, Table 2 [11–15]. The largest of these studies is a randomized phase 2 trial, which tested pembrolizumab at 2 mg/kg or 10 mg/kg vs. investigator’s choice chemotherapy. This trial included patients who progressed after ipilimumab and BRAF inhibition (if they were BRAF V600E mutant positive). The ORRs for patients receiving 2 and 10 mg/kg pembrolizumab were 21% and 25% respectively vs. 4% in the chemotherapy group, and the six-month progression-free survival (PFS) rates were 34% and 38% vs. 16%, respectively [14]. These data formed the basis of the recent FDA approval of this agent. The results of treatment with anti-PD-L1 have also been reported for 43 patients with advanced melanoma, with a similar ORR of 30%, and an additional subset of patients demonstrating prolonged disease stabilization [16] VSports app下载.
Table 2.
Weighted averages of reported objective response rates and number of patients(n) with multiple different types of advanced cancers treated with PD-1/PD-L1 blockade.
| anti-PD-1 | anti-PD-L1 | |||||
|---|---|---|---|---|---|---|
| Nivolumab | Pembrolizumab | Pidilizumab | BMS-936559 | MEDI4736 | MPDL3280A | |
| Melanoma | 35% (424) 10,35,36 | 27% (653) 12–14 | 6% (85) 15 | 17% (52)11 | - | 30% (43) 16 |
| NSCLC | 19% (149) 10,17,18 | 21% (226)19,20 | - | 10% (49) 11 | 16% (58) 21 | 23% (53) 16 |
| RCC | 20% (292) 10,22,23 | - | - | 12% (17) 11 | - | 14% (56) 16 |
| Bladder | - | 24% (29) 24 | - | - | - | 26% (65) 25 |
| Prostate | 0% (17) 10 | - | - | - | - | - |
| Hodgkin lymphoma | 87% (23) 26 | 53% (15) 27 | - | - | - | - |
| Ovarian | 23% (15) 29 | - | - | 6% (17) 11 | - | - |
| Breast | - | 19% (27) 30 | - | - | - | 33% (9)31 |
| CRC | 1* (33) 8,10 | - | - | - | - | - |
| Gastric | - | 31% (29) 33 | - | - | - | - |
| SCCHN | - | 20% (56) 32 | - | - | 14% (22) 33 | - |
The one patient who demonstrated a response had a microsatellite instability (MSI)-high tumor
NSCLC
In total, results from ~550 NSCLC patients treated with anti-PD-1/PD-L1 have been reported, Table 2 [10,11,16–21]. In the initial reports on nivolumab, the patients were heavily pre-treated [10,17] V体育安卓版. Similar response rates of 20% were seen when pembrolizumab when administered to previously treated NSCLC patients [20], and these rates increased when enrollment predicated PD-L1(+) tumor status (see discussion below of PD-L1 expression as a biomarker) [19]. More recent data with nivolumab as a first line agent has been reported, and an ORR of 30% was observed [18].
Response rates with anti-PD-L1 agents have ranged from an ORR of 10% in patients treated with BMS936559 as the second line or higher [11], to an ORR of 23% in heavily pretreated patients treated with MPDL3280A [16]. With MEDI4736, the OR and disease control rates (DCR, OR + stable disease >12 weeks) were 16% and 35%, respectively, when patients were not stratified according to PD-L1 status [21]. At this time, BMS936559 is not being further developed for the treatment of NSCLC, while additional trials using MPDL3280A and MEDI4736 are in progress.
Genitourinary cancers
Genitourinary cancers treated to date with anti-PD-1 and –PD-L1 include RCC, bladder cancer, and prostate cancer, Table 2. Approximately 80% of the patients treated with PD-1 pathway blockade for RCC have been on trials using nivolumab. In patients who had previously failed treatment with VEGF-pathway targeting agents, the ORR was 21% [22]. Response rates did not vary much with a previously untreated population [23]. Reported response rates were slightly less among patients receiving BMS-936559 or MPDL3280A, with ORR of 12% and 14%, respectively [11,16].
Exciting results have also been observed for patients with advanced bladder cancer, with a durable ORR of 24% reported for patients whose tumors demonstrated ≥1% PD-L1 expression treated with pembroluzimab [24]. Among anti-PD-L1 agents, results from studies with MPDL3280A showed a comparable ORR of 26% [25]. Unfortunately, clinical activity was not seen in patients with prostate cancer [10].
"V体育平台登录" Other tumor types
Early results are available on some hematolymphoid malignancies, gynecologic malignancies, breast cancer, gastrointestinal cancers and squamous cell carcinoma of the head and neck, Table 2. Patients with relapsed, refractory Hodgkin lymphoma have demonstrated remarkable treatment responses to nivolumab, with an ORR of 87%, and a progression-free survival of 86% at 24 weeks [26] and to pembrolizumab with an ORR of 53% in early analysis [27]. A small number of patients with other hematolymphoid malignancies have also been treated [28]. Nivolumab has also demonstrated efficacy in a proportion of patients with relapsed platinum-resistant ovarian cancer, with an observed 23% ORR and a 54% DCR [29]. Similarly, multiple clinical trials are underway with PD-1 pathway blocking agents for patients with breast cancer, where patients with triple-negative breast cancers have demonstrated ORR as high as 33% [31]. Response rates in patients with colorectal carcinoma have been meager overall, unless the patients have microsatellite-unstable tumors [8,10]. As such, efforts are now focused on studying these agents in tumors that are microsatellite instability (MSI)-high. Responses have also been observed in patients with pancreatic and gastric carcinomas, as well as in patients with head and neck squamous cell carcinoma (HNSCC) [16,32–34], and further exploration of these findings is actively underway by a number of different groups.
Phase 3 clinical trials
Melanoma
The results of two Phase 3 clinical trials for anti-PD-1 monotherapy in patients with melanoma have recently been reported. The first employed nivolumab as a first-line therapy for 418 treatment-naïve patients with unresectable melanoma whose tumors were BRAF wild type. Patients were randomized to receive either nivolumab or chemotherapy with decarbazine. The one-year analysis demonstrated an overall survival of 73% for the nivolumab patients vs. 42% for those who received decarbazine (p<0.001) [35] and helped promote the selection of anti-PD-1 as a treatment for patients with metastatic melanoma that is BRAF-wild type. The second phase 3 trial compared nivolumab to chemotherapy (decarbazine or carboplatin/paclitaxel) in 405 patients with advanced, metastatic melanoma [36]. Unlike the aforementioned trial, these patients had all been pre-treated with ipilimumb. In addition, a minority had also received a BRAF inhibitor. A 3-fold higher ORR was seen in the nivolumab group vs. the chemotherapy group (32% vs 11%).
NSCLC
Phase 3 trials of anti-PD-1 monotherapy have been launched for patients with NSCLC whose tumors are PD-L1+. These include two trials of pembroluzimab vs. chemotherapy (NCT02220894, NCT01905657) for both untreated and previously-treated patients, and a nivolumab vs. investigator’s choice chemotherapy trial for previously untreated patients (NCT02041533).
Immune-related adverse events
PD-1/PD-L1 checkpoint blockade is normally well-tolerated. Drug-related adverse events that are common to both anti-PD-1 and –PD-L1 agents include pruritis, fatigue, and loss of appetite. Immune related-adverse events (irAE) such as dermatitis, hypophesitis, colitis, and hepatitis have been reported for this class of agents, and in general are managed with corticosteroids and when essential, interruption of treatment. In the initial reports on nivolumab and BMS-936559, 14% and 9% of the patients developed grade 3 or 4 drug-related adverse events, respectively. Notably, three patients receiving nivolumab died from pneumonitis, and guidelines for identification, early intervention and management have been developed [37]. Since these initial reports, the overall incidence of irAEs has remained relatively constant, and is essentially the same between anti-PD-L1 therapies and anti-PD-1. However, the types of irAE differ, with no reported cases of pneumonitis or colitis observed in patients treated with anti-PD-L1 [16,25]. A difference in side effect and safety profile between the agents is certainly possible, given the differences in unblocked co-receptor interactions and, in some cases, differences in the isotype of antibodies. The latter can impact upon the potential for cell-mediated cytotoxicities, for example, IgG1 isotype antibodies more readily facilitate antibody-dependent cell-mediated cytotoxicity and complement-dependent cell-mediated cytotoxicity than those that are of the IgG4 isotype [38].
PD-L1 expression as a biomarker (VSports最新版本)
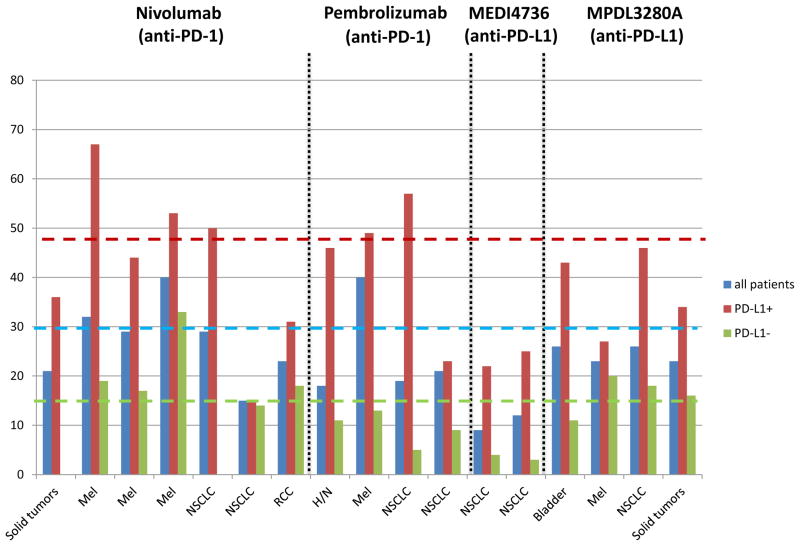
One of the key observations in these studies has been the association between PD-L1 expression in the tumor microenvironment and the response to therapy. This feature was first highlighted by Topalian et al, in 2012, who observed that out of 42 patients studied with multiple different solid tumor types, 36% who had PD-L1 detected on the surface of tumor cells by immunohistochemistry demonstrated an OR to nivolumab, while no patients who were PD-L1 (−) demonstrated a response [10]. In a follow up study, archival pre-treatment specimens were assayed for factors beyond tumor cell PD-L1 expression, including PD-L1 expression on infiltrating immune cells, PD-1 and PD-L2 expression, and simply the presence of a host immune response to tumor. Tumor cell PD-L1 expression remained the single feature most highly correlated with response [39]. This general observation of an association between PD-L1 expression and response to therapy has remained remarkably constant in the studies that have followed, irrespective of the solid tumor type studied, number of pre-treatment samples studied, IHC method or antibody used, agent tested (anti-PD-1 or anti-PD-L1), threshold of “positivity” (most often ≥1% or ≥5%), or even cell type scored (tumor cell vs. infiltrating immune cells) for PD-L1 expression. When the reported studies on solid tumors are summarized, 1400 patients have been assayed, and an average of 45% of patients who are PD-L1+ demonstrate an OR, Figure 1. Notably, 15% of PD-L1 (−) patients have also demonstrated responses. These collective results have mechanistic implications for this class of therapies, though the high proportion of PD-L1 (−) patients demonstrating an OR argues against the use of PD-L1 expression by IHC as a sole biomarker for patient selection.
Figure 1. Association of PD-L1 expression in pre-treatment tumor specimens with objective response to anti-PD-1/PD-L1 therapy.
Numerous studies in multiple tumor types have demonstrated the constant finding that PD-L1 expression enriches for response to anti-PD-1/PD-L1. The weighted average of the ORR across reported studies for patients whose tumors were tested for PD-L1 is 29% (blue dotted line), and if the specimen is PD-L1 (+), this increases to 48% (red dotted line). A significant proportion of PD-L1(−) patients also respond (green line). (Ref. from left to right 10, 40, 41, 35, 18, 17, 22, 32, 42, 43, 44, 33, 21, 25, 45, 16, 16).
"V体育2025版" FDA approval
These data have led to the US Food and Drug Administration’s (FDA) approval in 2014 of pembroluzimab and nivolumab for the treatment of advanced melanoma. Specifically, both are approved for patients who are refractory to ipilimumab and BRAF inhibitors (if the patient’s tumor harbors a BRAF-mutation). In early 2015, the FDA also extended the approved use of Nivolumab to include NSCLC patients who have failed platinum-based chemotherapies. Breakthrough designations have also been granted for the clinical development of nivolumab for resistant Hodgkin lymphoma and MPDL2380a in advanced bladder cancer. The approval of anti-PD-1 and PD-L1 therapy for additional tumor types is anticipated in the very near future. One or more of the IHC assays for PD-L1 detection may also become FDA approved.
Future directions
Potential differences in either efficacy or safety profiles between the agents blocking PD-1 and those blocking PD-L1 will likely require head-to-head trials before being fully elucidated. Future clinical development of either agent will involve identifying additional tumor types likely to respond to therapy, e.g., Merkel cell carcinoma [46] and cervical carcinoma [47]. Combination with other checkpoint agents has also demonstrated remarkable results, in the case of nivolumab and ipilimumab (anti-CTLA-4) for the treatment of advanced melanoma [48] and RCC [49], though further development may be required to reduce the intensity of immune-related adverse events observed with this specific combination. Results of trials of anti-PD-1/PD-L1 combined with other checkpoint agents such as LAG3 and TIM3 are eagerly anticipated, and added benefit may even be realized by combining anti-PD-1 and anti-PD-L1. Additional combinations include the addition of cancer vaccines, targeted inhibitors, and traditional chemotherapies. Efforts are also focused on the identification of additional factors beyond PD-L1 expression, such as cytotoxic T-lymphocyte density [50] or genomic signatures [51], which can aid in predicting response or resistance to anti-PD-1 or PD-L1 monotherapies. A more complete understanding of the complex interplay between the host and the tumor will permit a fuller realization of the anti-tumor effects of this exciting new class of agents.
Highlights.
Remarkable and durable anti-tumor activity with several anti-PD-1/PD-L1 antibodies
FDA approval for anti-PD-1 antibodies for advanced melanoma and NSCLC
PD-L1 expression in pre-treatment tumor enriches for response to anti-PD-1/PD-L1
Future studies will focus on combining other therapies with anti-PD-1/PD-L1
Footnotes
Disclosures: JMT receives research support from Bristol-Myers Squibb and is a member of advisory boards for Bristol-Myers Squibb.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
"VSports注册入口" References
- 1.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Hongo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI (V体育ios版)] [PubMed] [Google Scholar]
- 2.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 3.Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, et al. Expression of programmed death 1 ligands by murine T-cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 4.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishida M, Iwai Y, Tanaka Y, Okazaki T, Freeman GJ, Minato N, Honjo T. Differential expression of PD-L1 and PD-L2, ligands for an inhibitory receptor PD-1, in the cells of lymphohematopoietic tissues. Immunol Lett. 2002;84:57–62. doi: 10.1016/s0165-2478(02)00142-6. [DOI] [PubMed] [Google Scholar]
- 6.Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishiwata K, Watanabe N, Guo M, Tomihara K, Brumlik MJ, Yagita H, Pardoll D, Chen L, Shin T. Costimulator B7-DC attenuates strong Th2 responses induced by Nippostrongylus brasiliensis. J Immunol. 2010;184:2086–2094. doi: 10.4049/jimmunol.0804051. [V体育平台登录 - DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI (V体育官网)] [PMC free article] [PubMed] [Google Scholar]
- 9.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the United States, national cancer institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 10.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI (V体育平台登录)] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13**.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, Weber JS, Joshua AM, Hwu WJ, Gangadhar TC, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: A randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. This article reports on a trial with pembrolizumab (anti-PD-1) in ipilimumab-refractory patients. The presented findings contributed to FDA approval of pembrolizumab in September 2014. [V体育ios版 - DOI] [PubMed] [Google Scholar]
- 14.Dummer R, Daud A, Puzanov I, Hamid O, Schadendorf D, Robert C, Schachter J, Pavlick A, Gonzalez R, Hodi FS, et al. A randomized controlled comparison of pembrolizumab and chemotherapy in patients with ipilimumab-refractory melanoma. J Transl Med. 2015;13:2062. [Google Scholar]
- 15.Atkins MB, Kudchadkar RR, Sznol M, McDermott DF, Lotem M, Schachter J, Wolchok JD, Urba WJ, Kuzel T, Schuchter LM, et al. Phase 2, multicenter, safety and efficacy study of pidilizumab in patients with metastatic melanoma [abstract] J Clin Oncol (Meeting Abstracts) 2014;32:9001. [Google Scholar (V体育2025版)]
- 16**.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. In this study, MPDL3280A (anti-PD-L1) demonstrated anti-tumor activity in a number of different solid tumor types. Responses correlated with PD-L1 expression on infiltrating immune cells in the pre-treatment tumor specimens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brahmer JR, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, Powderly JD, Heist RS, Carvajal RD, Jackman DM, et al. Nivolumab (anti-PD-1, BMS-936558, ONO-4538) in patients (pts) with advanced non-small-cell lung cancer (NSCLC): Survival and clinical activity by subgroup analysis [abstract] J Clin Oncol (Meeting Abstracts) 2014;32:8112. [Google Scholar]
- 18.Gettinger SN, Shepherd FA, Antonia SJ, Brahmer JR, Chow LQM, Juergens RA, Borghaei H, Shen Y, Harbison C, Alaparthy S, et al. First-line nivolumab (anti-PD-1; BMS-936558, ONO-4538) monotherapy in advanced NSCLC: Safety, efficacy, and correlation of outcomes with PD-L1 status [abstract] J Clin Oncol (Meeting Abstracts) 2014;32:8024. [V体育ios版 - Google Scholar]
- 19.Rizvi NA, Garon EB, Patnaik A, Gandhi L, Leighl NB, Balmanoukian AS, Goldman JW, Eder JP, Johnson E, Blumenschein GR, et al. Safety and clinical activity of MK-3475 as initial therapy in patients with advanced non-small cell lung cancer (NSCLC) [abstract] J Clin Oncol (Meeting Abstracts) 2014;32:8007. [Google Scholar]
- 20.Garon EB, Leighl NB, Rizvi NA, Blumenschein GR, Balmanoukian AS, Eder JP, Goldman JW, Hui R, Soria JC, Gangadhar TC, et al. Safety and clinical activity of MK-3475 in previously treated patients (pts) with non-small cell lung cancer (NSCLC) [abstract] J Clin Oncol (Meeting Abstracts) 2014;32:8020. [VSports - Google Scholar]
- 21.Brahmer JR, Rizvi NA, Lutzky J, Khleif S, Blake-Haskins A, Li X, Robbins PB, Vasselli J, Ibrahim RA, Joseph S, et al. Clinical activity and biomarkers of MEDI4736, an anti-PD-L1 antibody, in patients with NSCLC [abstract] J Clin Oncol (Meeting Abstracts) 2014;32:8021. [Google Scholar]
- 22.Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, Vaishampayan UN, Drabkin HA, George S, Logan TF, et al. Nivolumab for metastatic renal cell carcinoma: Results of a randomized phase II trial. J Clin Onco. 2014 doi: 10.1200/JCO.2014.59.0703. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choueiri TK, Fishman MN, Escudier BJ, Kim JJ, Kluger HM, Stadler WM, Perez-Garcia LJ, McNeel DG, Curti BD, Harrison MR, et al. Immunomodulatory activity of nivolumab in previously treated and untreated metastatic renal cell carcinoma (mRCC): Biomarker-based results from a randomized clinical trial [abstract] J Clin Oncol (Meeting Abstracts) 2014;32:5012. [Google Scholar]
- 24.Plimack ER, Gupta S, Bellmunt J, Berger R, Montgomery B, Gonzalez EJ, Pulini J, Dolled-Filhart M, Emancipator K, Pathiraja K, et al. Phase 1B study of pembrolizumab (pembro; MK-3475) in patients (pts) with advanced urothelial tract cancer [abstract] Ann Oncol (Meeting Abstracts) 2014;25:LBA23A. [Google Scholar]
- 25.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 26**.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, et al. PD-1 blockade with nivolumab in relapsed or refractory hodgkin’s lymphoma. N Engl J Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. Phase I study for patients with relapsed/refractory Hodgkin lymphoma treated with nivolumab (anti-PD-1), showing clinical activity in the majority of patients treated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moskowitz CH, Ribrag V, Michot JM, Martinelli G, Zinzani PL, Gutierrez M, DeMaeyer G, Jacob AG, Giallella K, Anderson JW, et al. PD-1 blockade with the monoclonal antibody pembrolizumab (MK-3475) in patients with classical hodgkin lymphoma after brentuximab vedotin failure: Preliminary results from a phase 1b study (KEYNOTE-013) [abstract] Blood (Meeting Abstracts) 2014;124:290. [Google Scholar]
- 28.Lesokhin AM, Ansell SM, Armand P, Scott EC, Halwani A, Gutierrez M, Millenson MM, Cohen AD, Schuster SJ, Lebovic D, et al. Preliminary results of a phase I study of nivolumab (BMS-936558) in patients with relapsed or refractory lymphoid malignancies [abstract] Blood (Meeting Abstracts) 2014;124:291. [Google Scholar]
- 29.Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Matsumura N, Abiko K, Baba T, Yamaguchi K, Ueda A, et al. Efficacy and safety of anti-PD-1 antibody (nivolumab: BMS-936558, ONO-4538) in patients with platinum-resistant ovarian cancer [abstract] J Clin Oncol (Meeting Abstracts) 2014;32:5511. [Google Scholar]
- 30.Nanda R, Chow L, Dees E, Berger R, Gupta S, Geva R, Pusztai L, Dolled-Filhart M, Emancipator K, Gonzalez EJ, et al. A phase Ib study of pembrolizumab (MK-3475) in patients with advanced triple-negative breast cancer. Paper presented at: 2014 San Antonio Breast Cancer Symposium; 2014 December 9–13; San Antonio, Texas. [Google Scholar (VSports在线直播)]
- 31.Emens L, Braiteh F, Cassier P, DeLord JP, Eder JP, Shen X, Xiao Y, Wang Y, Hedge PS, Chen DS, et al. Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer. Paper presented at: 2014 San Antonio Breast Cancer Symposium; 2014 December 9–13; San Antonio, Texas. [Google Scholar (V体育官网入口)]
- 32.Seiwert TY, Burtness B, Weiss J, Gluck I, Eder JP, Pai SI, Dolled-Filhard M, Emancipator K, Pathiraja K, Gause C, et al. A phase Ib study of MK-3475 in patients with human papillomavirus (HPV)-associated and non-HPV-associated head and neck (H/N) cancer [abstract] J Clin Oncol (Meeting Abstracts) 2014;32:6011. [Google Scholar]
- 33.Segal NH, Antonia SJ, Brahmer JR, Maio M, Blake-Haskins A, Li X, Vasselli J, Ibrahim RA, Lutzky J, Khleif S, et al. Preliminary data from a multi-arm expansion study of MEDI4736, an anti-PD-L1 antibody [abstract] J Clin Oncol (Meeting Abstracts) 2014;32:3002. [Google Scholar]
- 34.Muro K, Bang Y, Shankaran V, Geva R, Catenacci DVT, Gupta S, Eder JP, Berger R, Gonzalez EJ, Pulini J, et al. Phase 1b study of pembrolizumab (pembro; MK-3475) in patients (pts) with advanced gastric cancer [abstract] Ann Oncol (Meeting Abstracts) 2014;25:LBA15A. [Google Scholar]
- 35.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [V体育安卓版 - DOI] [PubMed] [Google Scholar]
- 36.Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH, Jr, Lao CD, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015 Mar 17; doi: 10.1016/S1470-2045(15)70076-8. pii: S1470-2045(15)70076-8. [Epub ahead of print] ["VSports app下载" DOI] [PubMed] [Google Scholar]
- 37.Gangadhar TC, Vonderheide RH. Mitigating the toxic effects of anticancer immunotherapy. Nature. 2014;11:91–99. doi: 10.1038/nrclinonc.2013.245. [DOI] [PubMed] [Google Scholar]
- 38.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer--response. Clin Cancer Res. 2013;19:5542. doi: 10.1158/1078-0432.CCR-13-2234. ["VSports最新版本" DOI] [PMC free article] [PubMed] [Google Scholar]
- 39**.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. This article studied pre-treatment tumor specimens from patients treated on the first landmark trial with anti-PD-1[10]. Out of the multiple factors analyzed, PD-L1 expression by tumor was the single factor most associated with response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weber JS, Kudchadkar RR, Yu B, Gallenstein D, Horak CE, Inzunza HD, Zhao X, Matrinez AJ, Gibney G, Kroeger J, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31:4311–4318. doi: 10.1200/JCO.2013.51.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grosso J, Horak CE, Inzunza D, Cardona DM, Simon JS, Gupta AK, Sankar V, Park JS, Kollia G, Taube JM, et al. Association of tumor PD-L1 expression and immune biomarkers with clinical activity in patients (pts) with advanced solid tumors treated with nivolumab (anti-PD-1; BMS-936558; ONO-4538) [abstract] J Clin Oncol (Meeting Abstracts) 2013;31:3016. [Google Scholar (VSports app下载)]
- 42.Kefford R, Ribas A, Hamid O, Robert C, Daud A, Wolchok JD, Joshua AM, Hodi S, Gangadhar TC, Hersey P, et al. Clinical efficacy and correlation with tumor PD-L1 expression in patients (pts) with melanoma (MEL) treated with the anti-PD-1 monoclonal antibody MK-3475 [abstract] J Clin Oncol (Meeting Abstracts) 2014;32:3005. [VSports - Google Scholar]
- 43.Gandhi L, Balmanoukian A, Hui R, Hamid O, Rizvi NA, Leigh N, Gubens M, Goldman JW, Lubiniecki GM, Emancipator K, et al. MK-3475 (anti-PD-1 monoclonal antibody) for non-small cell lung cancer (NSCLC): Antitumor activity and association with tumor PD-L1 expression [abstract CT105]. Paper presented at: American Association for Cancer Research Annual Meeting; 2014 April 5–9; San Diego, CA. 2014. [Google Scholar]
- 44.Garon EB, Leighl NB, Rizvi NA, Blumenschein GR, Balmanoukian AS, Eder JP, Goldman JW, Hui R, Soria JC, Cangadhar TC, et al. Safety and clinical activity of MK-3475 in previously treated patients (pts) with non-small cell lung cancer (NSCLC) [abstract] J Clin Oncol (Meeting Abstracts) 2014;32:8020. [Google Scholar]
- 45.Hamid O, Sosman JA, Lawrence DP, Sullivan RJ, Ibrahim N, Kluger HM, Boasberg PD, Flaherty K, Hwu P, Balinger M, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic melanoma (mM) [abstract] J Clin Oncol (Meeting Abstracts) 2013;31:9010. [Google Scholar (VSports在线直播)]
- 46.Lipson EJ, Vincent JG, Loyo M, Kagohara LT, Luber BS, Wang H, Xu H, Nayar SK, Wang TS, Sidransky D, et al. PD-L1 expression in the Merkel cell carcinoma microenvironment: association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol Res. 2013;1:54–63. doi: 10.1158/2326-6066.CIR-13-0034. [VSports注册入口 - DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karim R, Jordanova ES, Piersma SJ, Kenter GG, Chen L, Boer JM, Melief CJ, van der Burg SH. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res. 2009;15:6341–6347. doi: 10.1158/1078-0432.CCR-09-1652. ["VSports app下载" DOI] [PubMed] [Google Scholar]
- 48**.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. Seminal trial combining two checkpoint inhibitors (anti-CTLA-4 and –PD-1), resulting in marked antitumor activity accompanied by an increase in immune related adverse events. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hammers HJ, Plimack ER, Infante JR, Ernstoff MS, Rini BI, McDermott DF, Razak ARA, Pal SK, Voss RH, Sharma P, et al. Phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma (mRCC) [abstract] J Clin Oncol (Meeting Abstracts) 2014;32:4504. [Google Scholar]
- 50.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015 Mar 12; doi: 10.1126/science.aaa1348. pii: aaa1348. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]