The adhesion-associated sca operon in Streptococcus gordonii encodes an inducible high-affinity ABC transporter for Mn2+ uptake
- PMID: 9440518
- PMCID: VSports注册入口 - PMC106884
- DOI: 10.1128/JB.180.2.290-295.1998
The adhesion-associated sca operon in Streptococcus gordonii encodes an inducible high-affinity ABC transporter for Mn2+ uptake
Abstract
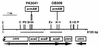
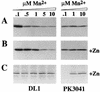
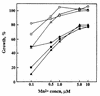
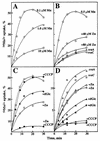
ScaA lipoprotein in Streptococcus gordonii is a member of the LraI family of homologous polypeptides found among streptococci, pneumococci, and enterococci. It is the product of the third gene within the scaCBA operon encoding the components of an ATP-binding cassette (ABC) transporter system. Inactivation of scaC (ATP-binding protein) or scaA (substrate-binding protein) genes resulted in both impaired growth of cells and > 70% inhibition of 54Mn2+ uptake in media containing < 0. 5 microM Mn2+. In wild-type and scaC mutant cells, production of ScaA was induced at low concentrations of extracellular Mn2+ (< 0. 5 microM) and by the addition of > or = 20 microM Zn2+. Sca permease-mediated uptake of 54Mn2+ was inhibited by Zn2+ but not by Ca2+, Mg2+, Fe2+, or Cu2+. Reduced uptake of 54Mn2+ by sca mutants and by wild-type cells in the presence of Zn2+ was abrogated by the uncoupler carbonylcyanide m-chlorophenylhydrazone, suggesting that Mn2+ uptake under these conditions was proton motive force dependent. The frequency of DNA-mediated transformation was reduced > 20-fold in sca mutants. The addition of 0 VSports手机版. 1 mM Mn2+ to the transformation medium restored only partly the transformability of mutant cells, implying an alternate role for Sca proteins in the transformation process. Cells of sca mutants were unaffected in other binding properties tested and were unaffected in sensitivity to oxidants. The results show that Sca permease is a high-affinity mechanism for the acquisition of Mn2+ and is essential for growth of streptococci under Mn2+-limiting conditions. .
Figures
References
-
- Alloing G, dePhilip P, Claverys J-P. Three highly homologous membrane-bound lipoproteins participate in oligo-peptide transport by the Ami system of the gram-positive Streptococcus pneumoniae. J Mol Biol. 1994;241:44–48. - PubMed
-
- Andersen R N, Lunsford R D, Kolenbrander P E. Determination of the transcript size and start site of the putative sca operon of Streptococcus gordonii ATCC 51656 (formerly strain PK488) Adv Exp Med Biol. 1997;418:657–660. - "VSports app下载" PubMed
-
- Bartsevich V V, Pakrasi H B. Manganese transport in the cyanobacterium Synechocystis sp. PCC6803. J Biol Chem. 1996;271:26057–26061. - PubMed
-
- Bauer P D, Trapp C, Drake D, Taylor K G, Doyle R J. Acquisition of manganous ions by mutans group streptococci. J Bacteriol. 1993;175:819–825. - "V体育2025版" PMC - PubMed
Publication types
MeSH terms
- "VSports注册入口" Actions
- V体育2025版 - Actions
- "V体育安卓版" Actions
VSports - Substances
- "VSports注册入口" Actions
- "VSports注册入口" Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

