GABAergic neurons in barrel cortex show strong, whisker-dependent metabolic activation during normal behavior
- PMID: 9204933
- PMCID: PMC6793834
- DOI: "VSports在线直播" 10.1523/JNEUROSCI.17-14-05509.1997
GABAergic neurons in barrel cortex show strong, whisker-dependent metabolic activation during normal behavior (VSports最新版本)
Abstract
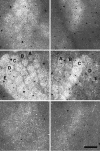
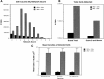
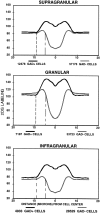
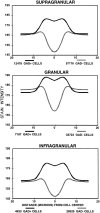
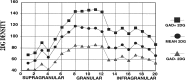
Electrophysiological data from the rodent whisker/barrel cortex indicate that GABAergic, presumed inhibitory, neurons respond more vigorously to stimulation than glutamatergic, presumed excitatory, cells. However, these data represent very small neuronal samples in restrained, anesthetized, or narcotized animals or in cortical slices. Histochemical data from primate visual cortex, stained for the mitochondrial enzyme cytochrome oxidase (CO) and for GABA, show that GABAergic neurons are more highly reactive for CO than glutamatergic cells, indicating that inhibitory neurons are chronically more active than excitatory neurons but leaving doubt about the short-term stimulus dependence of this activation. Taken together, these results suggest that highly active inhibitory neurons powerfully influence relatively inactive excitatory cells but do not demonstrate directly the relative activities of excitatory and inhibitory neurons in the cortex during normal behavior. We used a novel double-labeling technique to approach the issue of excitatory and inhibitory neuronal activation during behavior. Our technique combines high-resolution 2-deoxyglucose (2DG), immunohistochemical staining for neurotransmitter-specific antibodies, and automated image analysis to collect the data VSports手机版. We find that putative inhibitory neurons in barrel cortex of behaving animals are, on average, much more heavily 2DG-labeled than presumed excitatory cells, a pattern not seen in animals anesthetized at the time of 2DG injection. This metabolic activation is dependent specifically on sensory inputs from the whiskers, because acute trimming of most whiskers greatly reduces 2DG labeling in both cell classes in columns corresponding to trimmed whiskers. Our results provide confirmation of the active GABAergic cell hypothesis suggested by CO and single-unit data. We conclude that strong activation of inhibitory cortical neurons must confer selective advantages that compensate for its inherent energy inefficiency. .
Figures






References
-
- Agmon A, Connors BW. Correlation between intrinsic firing patterns and thalamocortical synaptic responses of neurons in mouse barrel cortex. J Neurosci. 1992;12:319–329. - PMC (V体育官网入口) - PubMed
-
- Armstrong-James M. The nature and plasticity of sensory processing within adult rat barrel cortex. In: Jones EG, Diamond IT, editors. Cerebral cortex. Plenum; New York: 1995. pp. 333–373.
-
- Beitz AJ, Larson AA, Monaghan P, Altschuler RA, Mullett MM, Madl JE. Immunohistochemical localization of glutamate, glutaminase, and aspartate aminotransferase in neurons of the pontine nuclei of the rat. Neuroscience. 1986;17:741–753. - VSports在线直播 - PubMed
-
- Bracewell RN. The Fourier transform and its applications, 2nd Ed. McGraw-Hill; New York: 1986.
-
- Bush PC, Sejnowski TJ. Effects of inhibition and dendritic saturation in simulated neocortical pyramidal cells. J Neurophysiol. 1994;71:2183–2193. - PubMed
Publication types
- Actions (VSports注册入口)
MeSH terms
- Actions (VSports)
- Actions (VSports app下载)
- "VSports app下载" Actions
- VSports手机版 - Actions
Substances
- Actions (V体育安卓版)
Grants and funding
LinkOut - more resources
Full Text Sources
