Loss of functional beta 2-microglobulin in metastatic melanomas from five patients receiving immunotherapy
- PMID: 8537970
- PMCID: PMC2248456
- DOI: 10.1093/jnci/88.2.100
"VSports注册入口" Loss of functional beta 2-microglobulin in metastatic melanomas from five patients receiving immunotherapy
Abstract
Background: In a subset of patients with metastatic melanoma, T lymphocytes bearing the cell-surface marker CD8 (CD8+ T cells) can cause the regression of even large tumors VSports手机版. These antitumor CD8+ T cells recognize peptide antigens presented on the surface of tumor cells by major histocompatibility complex (MHC) class I molecules. The MHC class I molecule is a heterodimer composed of an integral membrane glycoprotein designated the alpha chain and a noncovalently associated, soluble protein called beta2-microglobulin (beta 2m). Loss of beta 2m generally eliminates antigen recognition by antitumor CD8+ T cells. .
Purpose: We studied the loss of beta 2m as a potential means of tumor escape from immune recognition in a cohort of patients receiving immunotherapy V体育安卓版. .
Methods: We successfully grew 13 independent tumor cell cultures from tumor specimens obtained from 13 patients in a cohort of 40 consecutive patients undergoing immunotherapy for metastatic melanoma and for whom tumor specimens were available V体育ios版. These cell lines, as well as another melanoma cell line (called 1074mel) that had been derived from tumor obtained from a patient in a cytokine-gene therapy study, were characterized in vitro cytofluorometrically for MHC class I expression and by northern and western blot analyses for messenger RNA (mRNA) and protein expression, respectively, and ex vivo by immunohistochemistry. .
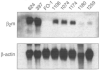
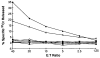
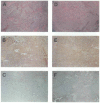
Results: After one melanoma cell line (1074mel) was found not to express functional beta 2m by cytofluorometric analysis, four (31%) of the 13 newly established melanoma cell lines were found to have an absolute lack of functional MHC class I expression. Northern blot analysis of RNA extracted from the five cell lines exhibiting no functional MHC class I expression showed that these cells contained normal levels of alpha-chain mRNA but variable levels of beta 2m mRNA. In addition, no immunoreactive beta 2m protein was detected by western blot analysis. When human beta 2m was transiently expressed with the use of a recombinant vaccinia virus, cell-surface MHC class I expression was reconstituted and the ability of these five cell lines to present endogenous antigens was restored VSports最新版本. Immunohistochemical staining of tumor sections revealed a lack of immunoreactive MHC class I in vivo, supporting the notion that the in vitro observations were not artifactual. Furthermore, archival tumor sections obtained from patients prior to immunotherapy were available from three patients and were found to be beta 2m positive. This result was consistent with the hypothesis that loss of beta 2m resulted from immunotherapy. .
Conclusions: These data suggest that the loss of beta 2m may be a mechanism whereby tumor cells can acquire immunoresistance. This study represents the first characterization of a molecular route of escape of tumors from immune recognition in a cohort of patients being treated with immunotherapy V体育平台登录. .
Figures



References
-
- Topalian SL. Cell transfer therapy: preclinical studies. In: DeVita VT Jr, Hellman S, Rosenberg SA, editors. Biologic therapy of cancer. 2nd ed. Lippincott; Philadelphia: 1995. pp. 467–86.
-
- Hoppe RT, Medeiros LJ, Warnke RA, Wood GS. CD8-positive tumor-infiltrating lymphocytes influence the long-term survival of patients with mycosis fungoides. J Am Acad Dermatol. 1995;32:448–53. - PubMed
-
- Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DF, Weber JS, et al. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2 [see comment citation in Medline] J Natl Cancer Inst. 1994;86:1159–66. - PubMed
-
- Ikarashi H, Fujita K, Takakuwa K, Kodama S, Tokunaga A, Takahashi T, et al. Immunomodulation in patients with epithelial ovarian cancer after adoptive transfer of tumor-infiltrating lymphocytes. Cancer Res. 1994;54:190–6. - PubMed
MeSH terms
- Actions (VSports最新版本)
- Actions (VSports手机版)
- "VSports app下载" Actions
- Actions (VSports app下载)
- "V体育2025版" Actions
- "VSports app下载" Actions
- VSports在线直播 - Actions
- "V体育安卓版" Actions
VSports手机版 - Substances
V体育安卓版 - Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
"VSports" Medical
Research Materials

