PDHA1 hyperacetylation-mediated lactate overproduction promotes sepsis-induced acute kidney injury via Fis1 lactylation (V体育ios版)
- PMID: 37479690
- PMCID: PMC10362039
- DOI: "VSports最新版本" 10.1038/s41419-023-05952-4
PDHA1 hyperacetylation-mediated lactate overproduction promotes sepsis-induced acute kidney injury via Fis1 lactylation
Abstract
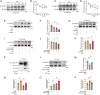
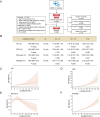
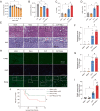
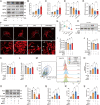
The increase of lactate is an independent risk factor for patients with sepsis-induced acute kidney injury (SAKI). However, whether elevated lactate directly promotes SAKI and its mechanism remain unclear. Here we revealed that downregulation of the deacetylase Sirtuin 3 (SIRT3) mediated the hyperacetylation and inactivation of pyruvate dehydrogenase E1 component subunit alpha (PDHA1), resulting in lactate overproduction in renal tubular epithelial cells. We then found that the incidence of SAKI and renal replacement therapy (RRT) in septic patients with blood lactate ≥ 4 mmol/L was increased significantly, compared with those in septic patients with blood lactate < 2 mmol/L. Further in vitro and in vivo experiments showed that additional lactate administration could directly promote SAKI VSports手机版. Mechanistically, lactate mediated the lactylation of mitochondrial fission 1 protein (Fis1) lysine 20 (Fis1 K20la). The increase in Fis1 K20la promoted excessive mitochondrial fission and subsequently induced ATP depletion, mitochondrial reactive oxygen species (mtROS) overproduction, and mitochondrial apoptosis. In contrast, PDHA1 activation with sodium dichloroacetate (DCA) or SIRT3 overexpression decreased lactate levels and Fis1 K20la, thereby alleviating SAKI. In conclusion, our results show that PDHA1 hyperacetylation and inactivation enhance lactate overproduction, which mediates Fis1 lactylation and exacerbates SAKI. Reducing lactate levels and Fis1 lactylation attenuate SAKI. .
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
"VSports最新版本" Figures


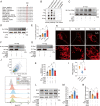
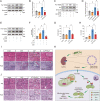
References (V体育ios版)
-
- Bellomo R, Kellum JA, Ronco C, Wald R, Martensson J, Maiden M, et al. Acute kidney injury in sepsis. Intensive Care Med. 2017;43:816–28. doi: 10.1007/s00134-017-4755-7. - "VSports注册入口" DOI - PubMed
-
- Zeng S, Lin J, Zhang W. Research progress of sepsis-induced acute kidney injury. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2018;30:703–7. - PubMed
-
- Honore PM, Jacobs R, Hendrickx I, Bagshaw SM, Joannes-Boyau O, Boer W, et al. Prevention and treatment of sepsis-induced acute kidney injury: an update. Ann Intensive Care. 2015;5:51–51. doi: 10.1186/s13613-015-0095-3. - VSports注册入口 - DOI - PMC - PubMed
-
- Park S, Jeon JH, Min BK, Ha CM, Thoudam T, Park BY, et al. Role of the pyruvate dehydrogenase complex in metabolic remodeling: differential pyruvate dehydrogenase complex functions in metabolism. Diabetes Metab J. 2018;42:270–81. doi: 10.4093/dmj.2018.0101. - DOI (VSports app下载) - PMC - PubMed
Publication types (V体育官网)
MeSH terms (V体育2025版)
- "V体育ios版" Actions
- VSports手机版 - Actions
- Actions (V体育安卓版)
- Actions (VSports手机版)
- "V体育2025版" Actions
"VSports" Substances
- Actions (V体育ios版)
- "V体育官网入口" Actions
LinkOut - more resources
Full Text Sources (VSports在线直播)
Medical
Molecular Biology Databases
VSports在线直播 - Miscellaneous

