VSports - FDX1-dependent and independent mechanisms of elesclomol-mediated intracellular copper delivery
- PMID: 36848556
- PMCID: PMC10013847
- DOI: 10.1073/pnas.2216722120
"VSports最新版本" FDX1-dependent and independent mechanisms of elesclomol-mediated intracellular copper delivery
Abstract
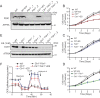
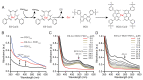
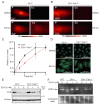
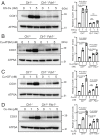
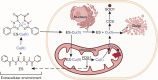
Recent studies have uncovered the therapeutic potential of elesclomol (ES), a copper-ionophore, for copper deficiency disorders. However, we currently do not understand the mechanism by which copper brought into cells as ES-Cu(II) is released and delivered to cuproenzymes present in different subcellular compartments. Here, we have utilized a combination of genetic, biochemical, and cell-biological approaches to demonstrate that intracellular release of copper from ES occurs inside and outside of mitochondria. The mitochondrial matrix reductase, FDX1, catalyzes the reduction of ES-Cu(II) to Cu(I), releasing it into mitochondria where it is bioavailable for the metalation of mitochondrial cuproenzyme- cytochrome c oxidase. Consistently, ES fails to rescue cytochrome c oxidase abundance and activity in copper-deficient cells lacking FDX1 VSports手机版. In the absence of FDX1, the ES-dependent increase in cellular copper is attenuated but not abolished. Thus, ES-mediated copper delivery to nonmitochondrial cuproproteins continues even in the absence of FDX1, suggesting alternate mechanism(s) of copper release. Importantly, we demonstrate that this mechanism of copper transport by ES is distinct from other clinically used copper-transporting drugs. Our study uncovers a unique mode of intracellular copper delivery by ES and may further aid in repurposing this anticancer drug for copper deficiency disorders. .
Keywords: FDX1; copper; cytochrome c oxidase; elesclomol; mitochondria V体育安卓版. .
Conflict of interest statement (V体育安卓版)
The authors have organizational affiliations to disclose: Vishal M. Gohil serves as a consultant to Engrail Therapeutics for their effort in developing elesclomol–copper as a therapeutic agent for Menkes disease. The authors have patent filings to disclose: S. S V体育ios版. and V. M. G. are inventors on the patent US 2021/0290571 A1 submitted by Texas A&M University entitled “Compositions for the Treatment of Copper Deficiency and Methods of Use. ”.
Figures
References
-
- Kaler S. G., Inborn errors of copper metabolism. Handb. Clin. Neurol. 113, 1745–1754 (2013). - V体育安卓版 - PMC - PubMed
-
- Papadopoulou L. C., et al. , Fatal infantile cardioencephalomyopathy with COX deficiency and mutations in SCO2, a COX assembly gene. Nat. Genet. 23, 333–337 (1999). - PubMed
-
- Baertling F., et al. , Mutations in COA6 cause cytochrome c oxidase deficiency and neonatal hypertrophic cardiomyopathy. Hum. Mutat. 36, 34–38 (2015). - PubMed
V体育官网入口 - Publication types
- "V体育官网" Actions
MeSH terms
- "VSports手机版" Actions
- V体育安卓版 - Actions
- Actions (V体育ios版)
- Actions (V体育ios版)
Substances
- V体育官网入口 - Actions
- "VSports app下载" Actions
- "VSports最新版本" Actions
"VSports手机版" Grants and funding
LinkOut - more resources
"VSports注册入口" Full Text Sources
"VSports" Research Materials

