VSports app下载 - Nrf2 Deficiency Exacerbated CLP-Induced Pulmonary Injury and Inflammation through Autophagy- and NF-κB/PPARγ-Mediated Macrophage Polarization
- PMID: 36497185
- PMCID: PMC9735993
- DOI: 10.3390/cells11233927
V体育平台登录 - Nrf2 Deficiency Exacerbated CLP-Induced Pulmonary Injury and Inflammation through Autophagy- and NF-κB/PPARγ-Mediated Macrophage Polarization
Abstract
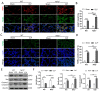
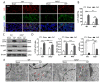
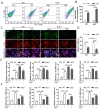
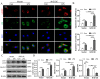
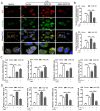
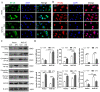
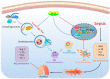
The balance between M1 and M2 macrophage polarization is involved in the regulation of pulmonary inflammation VSports手机版. Nuclear factor erythroid-derived 2-like 2 (Nfe2l2, also known as Nrf2), a nuclear transcription factor, is reported to play protective roles in acute lung injury (ALI) and inflammation, and increasing evidence indicates that the protective effects of Nrf2 are closely related to autophagy. This study aimed to explore whether Nrf2 is involved in sepsis-induced acute pulmonary injury and inflammation and in the role of macrophage polarization in the process. In the present study, sepsis patients, an Nrf2 knockout mouse that underwent cecal ligation and puncture (CLP), and lipopolysaccharide (LPS)-treated macrophage cell lines were employed to investigate the potential functions of Nrf2 in sepsis-induced lung injury and the underlying mechanisms. Clinical studies showed that the NRF2 mRNA level was inversely correlated with pulmonary inflammation and disease severity in patients with sepsis. Analyses in a CLP-treated Nrf2 knockout mouse model indicated that an Nrf2 deficiency promoted a CLP-induced increase in M1 macrophage polarization and apoptosis and inhibited CLP-induced upregulation of the autophagy level in lung tissues. Experiments in RAW264. 7 cells revealed that Nrf2 overexpression inhibited M1 macrophage polarization but promoted M2 macrophage polarization by improving the autophagy, and Nrf2 overexpression promoted PPARγ but inhibited NF-κB nuclear translocation. In conclusion, these results indicate that Nrf2 plays a protective role in sepsis-induced pulmonary injury and inflammation through the regulation of autophagy- and NF-κB/PPARγ-mediated macrophage polarization. .
Keywords: Nrf2; acute lung injury; autophagy; macrophage polarization; pulmonary inflammation V体育安卓版. .
Conflict of interest statement (V体育安卓版)
The authors declare no conflict of interest.
Figures



"VSports注册入口" References
-
- Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.-D., Coopersmith C.M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. - DOI - PMC - PubMed
-
- Shashaty M.G.S., Reilly J.P., Faust H.E., Forker C.M., Ittner C.A.G., Zhang P.X., Hotz M.J., Fitzgerald D., Yang W., Anderson B.J., et al. Plasma receptor interacting protein kinase-3 levels are associated with acute respiratory distress syndrome in sepsis and trauma: A cohort study. Crit. Care. 2019;23:235. doi: 10.1186/s13054-019-2482-x. - DOI - PMC - PubMed
-
- Liu Y., Zhou J., Luo Y., Li J., Shang L., Zhou F., Yang S. Honokiol alleviates LPS-induced acute lung injury by inhibiting NLRP3 inflammasome-mediated pyroptosis via Nrf2 activation in vitro and in vivo. Chin. Med. 2021;16:127. doi: 10.1186/s13020-021-00541-z. - "V体育官网" DOI - PMC - PubMed
MeSH terms
- Actions (V体育官网)
- V体育ios版 - Actions
- "V体育ios版" Actions
- Actions (VSports)
Substances
- Actions (VSports最新版本)
Grants and funding
V体育官网 - LinkOut - more resources
VSports - Full Text Sources
Medical
Miscellaneous

