Comprehensive analysis of cuproptosis-related genes in prognosis, tumor microenvironment infiltration, and immunotherapy response in gastric cancer
- PMID: 36462036
- PMCID: PMC11797702
- DOI: 10.1007/s00432-022-04474-4
V体育官网 - Comprehensive analysis of cuproptosis-related genes in prognosis, tumor microenvironment infiltration, and immunotherapy response in gastric cancer
V体育平台登录 - Abstract
Backgrounds: Cuproptosis is the most recently identified copper-dependent cell death form that influences tricarboxylic acid (TCA) cycle. However, the relationship between cuproptosis and clinical prognosis, tumor microenvironment infiltration (TME), and response to immunotherapy remains unclear VSports手机版. .
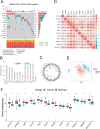
Methods: Single-sample gene-set enrichment analysis (ssGSEA) was employed to construct cuproptosisScore (cpS) and 1378 gastric cancer (GC) patients from five independent public datasets were classified into high- or low-cpS groups according to the median of cpS. Then the impacts of cuproptosis on tumor microenvironment infiltration (TME), biological function, response to immunotherapy, and clinical prognosis of GC were evaluated. RiskScore and nomogram were constructed using Lasso Cox regression algorithm to validate its predictive capability in GC patients V体育安卓版. .
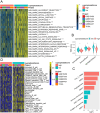
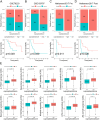
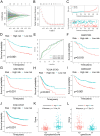
Results: Compared to patients with high cpS, patients with low cpS exhibited poorer prognosis, higher TNM stage, and stronger stromal activation. Meanwhile, the analysis of response to immunotherapy confirmed patients with high cpS could better benefit from immunotherapy and had a better susceptibility to chemotherapeutic drugs. Then, 9 prognosis-related signatures were collected based on differentially expressed genes (DEGs) of cpS groups. Finally, a riskScore model was constructed using the multivariate Cox (multi-Cox) regression coefficients of prognosis-related signatures and had an excellent capability of predicting 1-, 3-, and 5-year survival in GC patients V体育ios版. .
Conclusions: This study revealed the role of curproptosis in TME, response to immunotherapy, and clinical prognosis in GC, which highlighted the significant clinical implications of curproptosis and provided novel ideas for the therapeutic application of cuproptosis in GC. VSports最新版本.
Keywords: Cuproptosis; Gastric cancer; Immunotherapy; Prognosis; Tumor microenvironment. V体育平台登录.
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature. VSports注册入口.
"V体育ios版" Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures



"V体育官网入口" References
-
- Ajani JA, D’Amico TA, Almhanna K et al (2016) Gastric cancer, version 3.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 14(10):1286–1312. 10.6004/jnccn.2016.0137 (PMID: 27697982) - "V体育平台登录" PubMed
-
- Ajani JA, D’Amico TA, Bentrem DJ et al (2022) Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 20(2):167–192. 10.6004/jnccn.2022.0008 (PMID: 35130500) - "V体育ios版" PubMed
-
- Ambrus A, Adam-Vizi V (2018) Human dihydrolipoamide dehydrogenase (E3) deficiency: Novel insights into the structural basis and molecular pathomechanism. Neurochem Int 117:5–14. 10.1016/j.neuint.2017.05.018 (Epub 2017 Jun 2 PMID: 28579060) - PubMed
"VSports最新版本" MeSH terms
- Actions (VSports)
- "VSports" Actions
- Actions (V体育2025版)
- VSports app下载 - Actions
- "V体育官网" Actions
Grants and funding
- No. 2042022kf1127/the Fundamental Research Funds for the Central Universities
- grant no. ZNYB2021012/the Program of Excellent Doctoral (Postdoctoral) of Zhongnan Hospital of Wuhan University (VSports在线直播)
- Grant No. ZLYNXM202017/Wuhan University Zhongnan Hospital difficult disease diagnosis and treatment capacity improvement project (oncology)
- No. 2020CFB299/the Natural Science Foundation of Hubei Province
- Grant No. KYXM2022007/Special Project of knowledge Innovation of Wuhan Science and Technology Bureau (Dawning project)
LinkOut - more resources
Full Text Sources
Medical
V体育2025版 - Miscellaneous

