CST1 inhibits ferroptosis and promotes gastric cancer metastasis by regulating GPX4 protein stability via OTUB1
- PMID: 36369321
- PMCID: "V体育2025版" PMC9816059
- DOI: "VSports在线直播" 10.1038/s41388-022-02537-x
CST1 inhibits ferroptosis and promotes gastric cancer metastasis by regulating GPX4 protein stability via OTUB1
Abstract
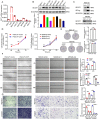
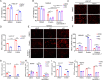
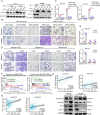
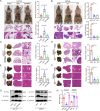
Metastasis is an important factor contributing to poor prognosis in patients with gastric cancer; yet, the molecular mechanism leading to this cell behavior is still not well understood. In this study, we explored the role of cysteine protease inhibitor SN (Cystatin SN, CST1) in promoting gastric cancer metastasis. We hypothesized that CST1 could regulate gastric cancer progression by regulating GPX4 and ferroptosis. Whole transcriptome sequencing suggested that the expression of CST1 was significantly increased in metastatic cancer, and high CST1 expression was correlated with a worse prognosis. Our data further confirmed that the overexpression of CST1 may significantly promote the migration and invasion of gastric cancer cells in vitro and enhance liver, lung, and peritoneal metastasis of gastric cancer in nude mice. Meanwhile, high expression of CST1 promoted the epithelial-mesenchymal transition (EMT) of gastric cancer cells. Mechanistically, a co-immunoprecipitation experiment combined with mass spectrometry analysis confirmed that CST1 could interact with GPX4, a key protein regulating ferroptosis. CST1 relieves GPX4 ubiquitination modification by recruiting OTUB1, improving GPX4 protein stability and reducing intracellular reactive oxygen species (ROS), thereby inhibiting ferroptosis and, in turn, promoting gastric cancer metastasis. Moreover, clinical data suggested that CST1 is significantly increased in peripheral blood and ascites of gastric cancer patients with metastasis; multivariate Cox regression model analysis showed that CST1 was an independent risk factor for the prognosis of gastric cancer patients. Overall, our results elucidated a critical pathway through which high CST1 expression protects gastric cancer cells from undergoing ferroptosis, thus promoting its progression and metastasis VSports手机版. CST1 may be used as a new oncological marker and potential therapeutic target for gastric cancer metastasis. .
© 2022. The Author(s).
Conflict of interest statement (V体育官网入口)
The authors declare no competing interests.
"VSports在线直播" Figures



V体育官网入口 - References
-
- Smyth EC, Moehler M. Late-line treatment in metastatic gastric cancer: today and tomorrow. Ther Adv Med Oncol. 2019;11:1758835919867522. doi: 10.1177/1758835919867522. - DOI (VSports最新版本) - PMC - PubMed
-
- Abrahamson M, Alvarez-Fernandez M, Nathanson CM. Cystatins. Biochem Soc Symp. 2003;70:179–99. doi: 10.1042/bss0700179. - "VSports app下载" DOI - PubMed
Publication types
MeSH terms
- VSports手机版 - Actions
- VSports手机版 - Actions
Substances
LinkOut - more resources
Full Text Sources
Medical
V体育安卓版 - Research Materials
Miscellaneous

