Target tumor microenvironment by innate T cells (VSports app下载)
- PMID: 36275727
- PMCID: PMC9582148
- DOI: 10.3389/fimmu.2022.999549
Target tumor microenvironment by innate T cells
Abstract
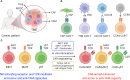
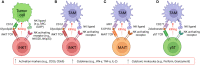
The immunosuppressive tumor microenvironment (TME) remains one of the most prevailing barriers obstructing the implementation of effective immunotherapy against solid-state cancers. Eminently composed of immunosuppressive tumor associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs) among others, the TME attenuates the effects of immune checkpoint blockade and adoptive cell therapies, mandating a novel therapy capable of TME remediation. In this review we explore the potential of three innate-like T cell subsets, invariant natural killer T (iNKT), mucosal-associated invariant T (MAIT) cells, and gamma delta T (γδT) cells, that display an intrinsic anti-TAM/MDSC capacity. Exhibiting both innate and adaptive properties, innate-like T cell types express a subset-specific TCR with distinct recombination, morphology, and target cell recognition, further supplemented by a variety of NK activating receptors. Both NK activating receptor and TCR activation result in effector cell cytotoxicity against targeted immunosuppressive cells for TME remediation. In addition, innate-like T cells showcase moderate levels of tumor cell killing, providing dual antitumor and anti-TAM/MDSC function. This latent antitumor capacity can be further bolstered by chimeric antigen receptor (CAR) engineering for recognition of tumor specific antigens to enhance antitumor targeting. In contrast with established CAR-T cell therapies, adoption of these innate-like cell types provides an enhanced safety profile without the risk of graft versus host disease (GvHD), due to their non-recognition of mismatched major histocompatibility complex (MHC) molecules, for use as widely accessible, allogeneic "off-the-shelf" cancer immunotherapy VSports手机版. .
Keywords: cell-based immunotherapy; gamma delta T (γδT) cell; innate T cell; invariant natural killer T (iNKT) cell; mucosal-associated invariant T (MAIT) cell; myeloid-derived suppressor cell (MDSC); tumor microenvironment (TME); tumor-associated macrophage (TAM) V体育安卓版. .
Copyright © 2022 Li, Wilson and Yang.
Conflict of interest statement
Y-RL and LY are inventors on patents relating to this article filed by UCLA. LY is a scientific advisor to AlzChem and Amberstone Biosciences, and a co-founder, stockholder, and advisory board member of Appia Bio. None of the declared companies contributed to or directed any of the research reported in this article V体育ios版. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
V体育ios版 - Figures

References (V体育2025版)
-
- Li Y-R, Yu Y, Kramer A, Hon R, Wilson M, Brown J, et al. . An ex vivo 3D tumor microenvironment-mimicry culture to study TAM modulation of cancer immunotherapy. Cells (2022) 11:1583. doi: 10.3390/cells11091583 - DOI (V体育ios版) - PMC - PubMed
Publication types
- Actions (V体育官网)
MeSH terms
- V体育官网入口 - Actions
- Actions (V体育ios版)
- "V体育安卓版" Actions
"VSports最新版本" Substances
- V体育ios版 - Actions
VSports注册入口 - LinkOut - more resources
Full Text Sources
Research Materials

