"V体育安卓版" Mucosal-associated invariant T cells predict increased acute graft-versus-host-disease incidence in patients receiving allogeneic hematopoietic stem cell transplantation
- PMID: 36180885
- PMCID: PMC9526319
- DOI: 10.1186/s12935-022-02703-x
Mucosal-associated invariant T cells predict increased acute graft-versus-host-disease incidence in patients receiving allogeneic hematopoietic stem cell transplantation
Abstract
Background: Mucosal-associated invariant T (MAIT) cells are innate-like T cells, some studies have reported that the number of circulating MAIT cells reduced in patients with acute graft-versus-host-disease (aGVHD) development VSports手机版. However, the role of donor MAIT cells on aGVHD development and subsequent functional change still remain unclear. .
Methods: The study recruited 86 patients with hematological malignancies who underwent allogeneic hematopoietic cell transplantation (HCT) from May 1, 2018 to June 30, 2019 V体育安卓版. MAIT cells, their subset, and cytokine levels were measured by flow cytometry. Gray's test was used to assess the impact of graft MAIT cell proportion and number on aGVHD incidence. The Cox proportional hazard model was used in the multivariate analysis. The comparison for continuous variables was assessed using Mann-Whitney analysis. RNA-sequencing was performed to investigate the possible molecular pathway changes. .
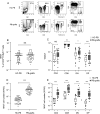
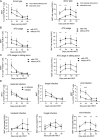
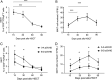
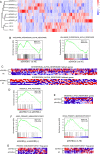
Results: Our study showed that the proportion of MAIT cells in grafts was not different from normal controls, but the CD4/8 subsets were altered. Taking the median of the proportion and number of MAIT cells in the graft as the threshold, the results showed that the incidence of grade B-D aGVHD in patients with MAIT cell proportion ≥ 3. 03% was significantly higher than that in patients with MAIT cell proportion < 3. 03% (56. 3%, 95% CI 37. 1-71. 2 versus 23. 1%, 95% CI 13. 8-46 V体育ios版. 2; P = 0. 038). The number of MAIT cells in the graft was not associated with aGVHD development (P = 0. 173), however, when the graft contained more CD4 positive, CD8 positive, and CD4/CD8 double-positive MAIT cells, the incidence of aGVHD was significantly increased (P = 0. 019, P = 0. 035 and P = 0. 027, respectively). Besides, reduced frequencies and counts of circulating MAIT cells were observed in patients with aGVHD when compared to patients without aGVHD, accompanied by enhanced production of Tumor necrosis factor-α, Interferon-γ and upregulated programmed death-1, CXC Chemokine Receptor-6 (CXCR6) and CD38 expression. Gene set enrichment analysis of MAIT cell RNA-seq data showed interferon-α response pathway upregulated in aGVHD patients when compared with patients without aGVHD and healthy controls. .
Conclusions: Our study shows that MAIT cells in grafts and peripheral blood are both closely related to the aGVHD development post allogeneic HCT VSports最新版本. Interferon-α response pathway perhaps is a critical regulation mechanism for the MAIT cell involvement in aGVHD development. .
Keywords: Cytokines; Graft versus host disease; Hematopoietic cell transplantation; MAIT cell. V体育平台登录.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


"VSports注册入口" References
-
- Dusseaux M, Martin E, Serriari N, Peguillet I, Premel V, Louis D, et al. Human MAIT cells are xenobiotic–resistant, tissue-targeted, CD161hi IL-17–secreting T cells. Blood. 2011;117(4):1250–1259. doi: 10.1182/blood-2010-08-303339. - "VSports手机版" DOI - PubMed
-
- McWilliam HE, Birkinshaw RW, Villadangos JA, McCluskey J, Rossjohn J. MR1 presentation of vitamin B-based metabolite ligands. Curr Opin Immunol. 2015;34:28–34. doi: 10.1016/j.coi.2014.12.004. - DOI (V体育2025版) - PubMed
-
- Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, et al. Selection of evolutionarily conserved mucosal–associated invariant T cells by MR1. Nature. 2003;422(6928):164–169. doi: 10.1038/nature01433. - "V体育安卓版" DOI - PubMed
Grants and funding
LinkOut - more resources
"V体育安卓版" Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

