"VSports注册入口" Liver organoids: an in vitro 3D model for liver cancer study
- PMID: 36085085
- PMCID: PMC9463833
- DOI: 10.1186/s13578-022-00890-8
"VSports在线直播" Liver organoids: an in vitro 3D model for liver cancer study
Abstract
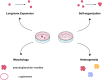
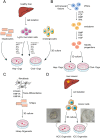
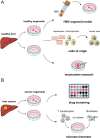
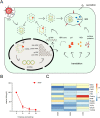
Primary liver cancer (PLC) is the second leading cause of cancer mortality worldwide, and its morbidity unceasingly increases these years. Hepatitis B virus (HBV) infection accounted for approximately 50% of hepatocellular carcinoma (HCC) cases globally in 2015 VSports手机版. Due to the lack of an effective model to study HBV-associated liver carcinogenesis, research has made slow progress. Organoid, an in vitro 3D model which maintains self-organization, has recently emerged as a powerful tool to investigate human diseases. In this review, we first summarize the categories and development of liver organoids. Then, we mainly focus on the functions of culture medium components and applications of organoids for HBV infection and HBV-associated liver cancer studies. Finally, we provide insights into a potential patient-derived organoid model from those infected with HBV based on our study, as well as the limitations and future applications of organoids in liver cancer research. .
Keywords: HBV infection; Liver organoid; Primary liver cancer V体育安卓版. .
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
"V体育官网" Figures
References
Publication types (VSports最新版本)
- VSports在线直播 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
V体育平台登录 - Other Literature Sources

