Co-delivery of phagocytosis checkpoint and STING agonist by a Trojan horse nanocapsule for orthotopic glioma immunotherapy (V体育官网入口)
- PMID: 35910792
- PMCID: PMC9330518 (VSports app下载)
- DOI: V体育平台登录 - 10.7150/thno.73104
"V体育ios版" Co-delivery of phagocytosis checkpoint and STING agonist by a Trojan horse nanocapsule for orthotopic glioma immunotherapy
Abstract
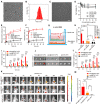
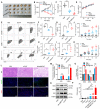
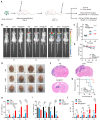
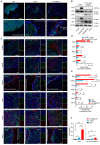
Rationale: Cancer immunotherapy has demonstrated significant antitumor activity in a variety of tumors; however, extensive infiltration of immunosuppressive tumor-associated macrophages (TAMs) in the glioblastoma (GBM) tumor microenvironment (TME) and the existence of the blood-brain barrier (BBB) might lead to failure of the checkpoint blockade therapy. Methods: Herein, we have developed a smart "Trojan horse" BBB-permeable nanocapsule termed "NAcp@CD47" to deliver anti-CD47 antibodies and stimulator of interferon genes (STING) agonists into GBM tissues in a stealth-like manner to reshaped the immune microenvironment by switching the phenotype of microglia and macrophages. Results: Both in vitro and in vivo studies demonstrate that NAcp@CD47 could effectively penetrate the BBB, increase the polarization of M1-phenotype TAMs, help reduce tumor immunosuppression, and inhibit the orthotopic GBM growth by phagocytosis of macrophages and microglia VSports手机版. Conclusions: Our findings indicate that the well-designed NAcp@CD47 not only enhances the phagocytosis of cancer cells but also efficiently enhance antitumor immunogenicity and reverses immune suppression to convert uninflamed "cold" tumors into "hot" tumors. .
Keywords: CD47; glioblastoma; immunotherapy; phagocytosis; tumor associated macrophages. V体育安卓版.
© The author(s).
Conflict of interest statement (V体育ios版)
Competing Interests: The authors have declared that no competing interest exists.
Figures (V体育平台登录)


References
-
- Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet. 2018;392:432–46. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. - PubMed
-
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. - PubMed
-
- Jain KK. A critical overview of targeted therapies for glioblastoma. Front Oncol. 2018;8:419. - VSports - PMC - PubMed
"VSports最新版本" Publication types
MeSH terms
- V体育ios版 - Actions
- VSports手机版 - Actions
- "V体育官网" Actions
- Actions (V体育官网入口)
- "VSports在线直播" Actions
V体育官网入口 - Substances
- Actions (V体育官网)

