Coordination of metal center biogenesis in human cytochrome c oxidase
- PMID: 35750769
- PMCID: VSports app下载 - PMC9232578
- DOI: "VSports最新版本" 10.1038/s41467-022-31413-1
Coordination of metal center biogenesis in human cytochrome c oxidase
Abstract (V体育安卓版)
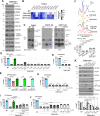
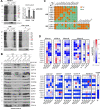
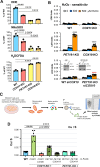
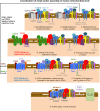
Mitochondrial cytochrome c oxidase (CcO) or respiratory chain complex IV is a heme aa3-copper oxygen reductase containing metal centers essential for holo-complex biogenesis and enzymatic function that are assembled by subunit-specific metallochaperones. The enzyme has two copper sites located in the catalytic core subunits. The COX1 subunit harbors the CuB site that tightly associates with heme a3 while the COX2 subunit contains the binuclear CuA site. Here, we report that in human cells the CcO copper chaperones form macromolecular assemblies and cooperate with several twin CX9C proteins to control heme a biosynthesis and coordinate copper transfer sequentially to the CuA and CuB sites. These data on CcO illustrate a mechanism that regulates the biogenesis of macromolecular enzymatic assemblies with several catalytic metal redox centers and prevents the accumulation of cytotoxic reactive assembly intermediates VSports手机版. .
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Yoshikawa S, et al. Redox-coupled crystal structural changes in bovine heart cytochrome c oxidase. Science. 1998;280:1723–1729. doi: 10.1126/science.280.5370.1723. - DOI (V体育2025版) - PubMed
Publication types
- VSports在线直播 - Actions
- "VSports app下载" Actions
MeSH terms
- VSports手机版 - Actions
- V体育2025版 - Actions
- Actions (V体育官网入口)
Substances (VSports app下载)
- Actions (V体育官网)
- V体育安卓版 - Actions
- "VSports最新版本" Actions
- VSports - Actions
- VSports注册入口 - Actions
Grants and funding (V体育安卓版)
LinkOut - more resources (VSports app下载)
Full Text Sources
"VSports最新版本" Molecular Biology Databases
Research Materials

