VSports在线直播 - Human gingival mesenchymal stem cells retain their growth and immunomodulatory characteristics independent of donor age
- PMID: 35749495
- PMCID: "V体育ios版" PMC9232118
- DOI: 10.1126/sciadv.abm6504 (V体育官网)
Human gingival mesenchymal stem cells retain their growth and immunomodulatory characteristics independent of donor age (V体育官网入口)
Abstract
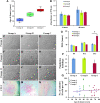
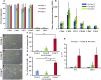
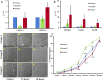
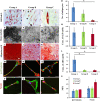
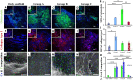
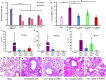
Aging has been reported to deteriorate the quantity and quality of mesenchymal stem cells (MSCs), which affect their therapeutic use in regenerative medicine VSports手机版. A dearth of age-related stem cell research further restricts their clinical applications. The present study explores the possibility of using MSCs derived from human gingival tissues (GMSCs) for studying their ex vivo growth characteristics and differentiation potential with respect to donor age. GMSCs displayed decreased in vitro adipogenesis and in vitro and in vivo osteogenesis with age, but in vitro neurogenesis remained unaffected. An increased expression of p53 and SIRT1 with donor age was correlated to their ability of eliminating tumorigenic events through apoptosis or autophagy, respectively. Irrespective of donor age, GMSCs displayed effective immunoregulation and regenerative potential in a mouse model of LPS-induced acute lung injury. Thus, we suggest the potential of GMSCs for designing cell-based immunomodulatory therapeutic approaches and their further extrapolation for acute inflammatory conditions such as acute respiratory distress syndrome and COVID-19. .
Figures
References
-
- Asumda F. Z., Age-associated changes in the ecological niche: Implications for mesenchymal stem cell aging. Stem Cell Res. Ther. 4, 47 (2013). - PMC (VSports在线直播) - PubMed
-
- Ma D., Ma Z., Zhang X., Wang W., Yang Z., Zhang M., Wu G., Lu W., Deng Z., Jin Y., Effect of age and extrinsic microenvironment on the proliferation and osteogenic differentiation of rat dental pulp stem cells in vitro. J. Endod. 35, 1546–1553 (2009). - PubMed
-
- Iohara K., Murakami M., Nakata K., Nakashima M., Age-dependent decline in dental pulp regeneration after pulpectomy in dogs. Exp. Gerontol. 52, 39–45 (2014). - PubMed
"VSports" MeSH terms
- VSports手机版 - Actions
LinkOut - more resources
Full Text Sources
Medical
V体育安卓版 - Research Materials
Miscellaneous

