Circ_0000064 promotes high glucose-induced renal tubular epithelial cells injury to facilitate diabetic nephropathy progression through miR-532-3p/ROCK1 axis (V体育ios版)
- PMID: 35291991
- PMCID: PMC8922934 (V体育平台登录)
- DOI: 10.1186/s12902-022-00968-x
Circ_0000064 promotes high glucose-induced renal tubular epithelial cells injury to facilitate diabetic nephropathy progression through miR-532-3p/ROCK1 axis
Abstract
Background: Circular RNA (circRNA) has been shown to mediate diabetic nephropathy (DN) development by regulating renal tubular epithelial cells (RTECs) injury VSports手机版. However, the role and mechanism of circ_0000064 in high glucose (HG)-induced RTECs injury have not been fully elucidated. .
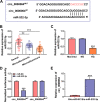
Methods: Human RTECs (HK-2) were exposed to HG to induce cell injury. Cell oxidative stress was assessed by detecting the levels of oxidative stress-markers. Moreover, cell proliferation and apoptosis were determined by CCK8 assay, EDU assay and flow cytometry V体育安卓版. The protein levels of proliferation markers, apoptosis markers and Rho-associated coiled-coil-containing kinase 1 (ROCK1) were measured using western blot analysis. Furthermore, quantitative real-time PCR was performed to assess the expression of circ_0000064, microRNA (miR)-532-3p and ROCK1. The interaction between miR-532-3p and circ_0000064 or ROCK1 was confirmed by dual-luciferase reporter assay and RNA pull-down assay. .
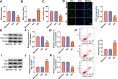
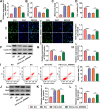
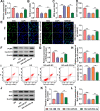
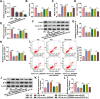
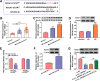
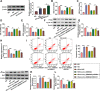
Results: Our results revealed that HG treatment could promote HK-2 cells oxidative stress, apoptosis, fibrosis, and inhibit proliferation. Circ_0000064 expression was increased in the serum of DN patients and HG-induced HK-2 cells, and silenced circ_0000064 could relieve HG-induced HK-2 cells injury. MiR-532-3p could be sponged by circ_0000064, and its overexpression also alleviated HG-induced HK-2 cells injury. Besides, the regulation of circ_0000064 knockdown on HG-induced HK-2 cells injury could be reversed by miR-532-3p inhibitor V体育ios版. Additionally, ROCK1 was a target of miR-532-3p, and its expression was inhibited by circ_0000064 knockdown. The inhibition effect of circ_0000064 knockdown on HG-induced HK-2 cells injury also could be reversed by overexpressing ROCK1. .
Conclusion: In summary, circ_0000064 knockdown might alleviate HG-induced HK-2 cells injury via regulating the miR-532-3p/ROCK1 axis, which provided a new perspective for DN treatment VSports最新版本. .
Keywords: Circ_0000064; Diabetic nephropathy; High glucose; ROCK1; miR-532-3p. V体育平台登录.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

References
-
- Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab. 2008;4(8):444–452. doi: 10.1038/ncpendmet0894. - "VSports" DOI - PubMed
-
- Usuelli V, La Rocca E. Novel therapeutic approaches for diabetic nephropathy and retinopathy. Pharmacol Res. 2015;98:39–44. doi: 10.1016/j.phrs.2014.10.003. - V体育2025版 - DOI - PubMed
"V体育官网" MeSH terms
- Actions (V体育ios版)
- V体育ios版 - Actions
- VSports - Actions
- Actions (VSports app下载)
- V体育官网入口 - Actions
Substances
- "VSports app下载" Actions
- Actions (VSports注册入口)
LinkOut - more resources
Full Text Sources
Medical

